Abstract
Subserved by a dense network of neuro-anatomical connections within the cerebrum, the cerebellum fulfills a crucial role in various motor, cognitive, and affective functions, and is located immediately below the skull. As a result, the cerebellum appears an interesting target for modern noninvasive stimulation techniques such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). This literature survey intends to give a short overview of what is currently known about noninvasive stimulation techniques applied to the cerebellum.
Introduction
Improving rehabilitation of brain functions by modulating the excitability of neurons with noninvasive techniques such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) is an exciting new research domain. Since (a) the cerebellothalamocortical pathways connect the cerebellum with the supratentorial regions subserving motor, associative, and affective functions and (b) the cerebellar circuitry is located immediately below the skull, the cerebellum might be a very promising target for noninvasive stimulation.1 We conducted a literature search to determine the potential value of cerebellar stimulation in different domains.
Working mechanisms of TMS and tDCS
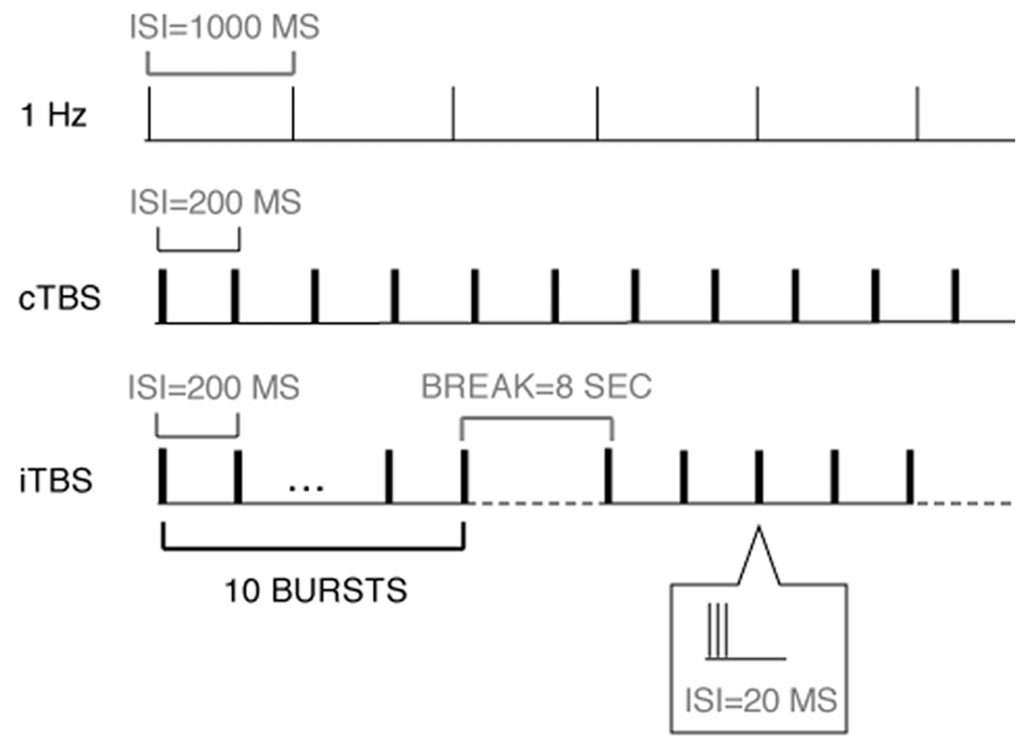
TMS is a non-invasive brain stimulation technique that offers the possibility of modulating the excitability and activity of specific brain areas.2 TMS uses a coil to produce a pulsed magnetic field inducing an electric field inside the brain (eddy currents). If the intensity of the eddy current exceeds a particular threshold, action potentials are generated in the stimulated neurons.3 TMS can be administered in several different ways (Figure 1). A single pulse of TMS can be used (single pulse TMS) or it can be administered in a repetitive manner at different frequencies (rTMS), and in different patterns (such as theta burst stimulation, TBS). When a single pulse is used, it is usually given at an intensity that can generate action potentials, which can temporarily interfere with the function of the targeted brain region. The different TMS paradigms all exert a specific effect on cerebellar (and cortical) excitability, exciting or inhibiting brain function.4
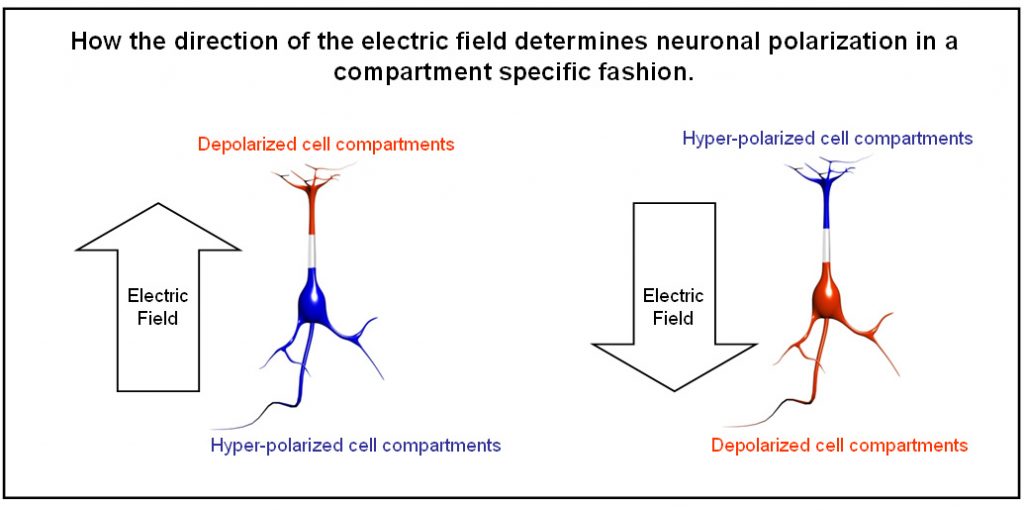
tDCS is another novel brain stimulation technique that uses two electrodes to induce a small electric current in the brain.6 Anodal and cathodal tDCS are associated with opposite directions in currents. As compared to TMS, tDCS does not generate action potentials in neurons but acts on the polarisation of cellular membranes (Figure 2). This means that tDCS modulates activity in active neurons but has probably little impact on purely resting neuronal population.7 The general rule is that anodal tDCS increases the excitability of neurons, whereas cathodal tDCS exerts the opposite effect. However, this is a simplication of the physiological mechanisms subserving the effects of tDCS. tDCS creates a difference of electric potential between two (or more) electrodes, which induces a shift in the membrane potential and therefore modifies the excitability of the neurons within the created electric field.7,8 Electric brain stimulation can also be achieved using alternating current and is called transcranial Alternating Current Stimulation (tACS). At low frequency, this will lead to alternating membrane potential changes following the current wave.9
However, little is known about the exact impact of electric/magnetic stimulation of the cerebellum since the cerebellum differs from cortical brain tissue in many respects, especially the cytoarchitecture. In addition, complex cerebellar folding greatly affects the changes in excitability which makes a prediction of the outcome very difficult.10 It is also challenging that the effects of cerebellar stimulation are difficult to measure in the cerebellum itself. The impact on cerebello-cortical mechanisms (such as cerebellar brain inhibition (CBI)) has to be monitored to evaluate the effectiveness of cerebellar stimulation.11,12
Anatomical cerebello-cerebral connections
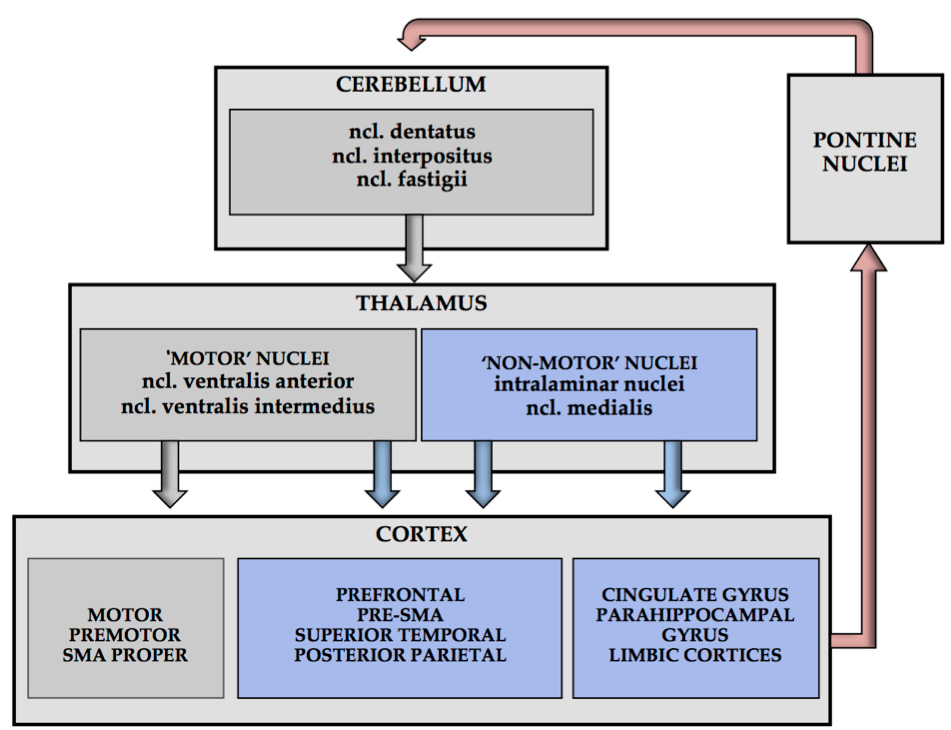
Neuroanatomical evidence has shown multiple crossed cerebello-cerebral connections, not only with the contralateral motor areas, but also with the associative cortices responsible for cognition and affect (Figure 3). Many functional imaging studies confirmed involvement of the cerebellum in a variety of motor, cognitive, and affective functions.13 However, several recent studies indicate that there are also non-crossing cerebello-cerebral pathways,14 and direct connections between both cerebellar hemispheres.15 Future studies should bear in mind that the functional connectivity of the cerebellum to the supratentorial regions is built on a complex network consisting of crossed and non-crossed pathways,14 supplemented with parallel connections between both cerebellar hemispheres.15
Findings of the literature survey
A literature survey (Electronic online databases: Web of Knowledge, ScienceDirect, PubMed, Medline; keywords: cerebell* AND tDCS OR transcranial direct current stimulation; cerebell* AND TMS OR transcranial magnetic stimulation) yielded 111 original studies using cerebellar TMS and 49 studies using cerebellar tDCS, covering a wide and extensive range of topics. Most studies applied stimulation in healthy subjects (TMS: n=81; tDCS: n=35) with a focus on probing functional connectivity with cerebellar TMS (n=28) and motor function with cerebellar tDCS (n=16). Other areas included cognition and affect, and some studies explored the effects of cerebellar stimulation in a clinical population (TMS: n=30; tDCS: n=14).
General
In general, the timing of the administration (together with therapy/assessment or not) and the type of TMS (single pulse TMS, low frequency rTMS, high frequency rTMS, intermittent TBS, continuous TBS) or tDCS (anodal, cathodal) stimulation is very important. These parameters determine which process will be affected, and in what way. In addition, the intensity of the stimulation may also be crucial, especially for TMS in which the intensities applied are frequently determined by the resting or the active motor threshold of the contralateral motor cortex. These intensities may vary greatly and probably have a differential impact on neuronal ring and functional connectivity. Moreover, it is important that the stimulation only affects the cerebellum and does not spread to the adjacent brainstem or to the visual cortex. With regard to cerebellar tDCS, the effect of the intensity of the current on neuronal excitability has not been systematically investigated. Due to the various cerebello-cerebral connections, the lateralisation of functions in the cerebral hemispheres, as well as the cerebello-spinal pathways, it is also important to carefully select the place of stimulation. With TMS the localisation is dependent on the positioning of the coil, the coil orientation, and the type of coil used. Several studies have indicated that the standard figure-of-eight coil may not be optimal for cerebellar stimulation due to the distance between the scalp and the cerebellar cortex. It is therefore important in future studies to use a double cone or a batwing coil, which are specifically designed for stimulating deeper brain regions and which eliminate the stimulation of the peripheral nerves that is observed in cerebellar stimulation with a figure-of-eight coil.3,4,11 For tDCS, the positioning of the electrodes and their size primarily determines which areas will be excited. The exact electrical pathways taken during tDCS are still unclear and more studies should be made to better characterise them.
Motor
The literature on motor function shows that the cerebellum is involved in movement, motor learning, motor adaptation, and even motor imagery. The cerebellum seems to be responsible for monitoring ongoing movements, and predicting future states, but also for detecting and correcting errors (state estimations).18 Interestingly, however, the complexity of the task at hand has a significant impact on the effect of cerebellar stimulation. The nature of the task also affects the outcome differently (e.g. cerebellar stimulation has a different impact on motor movement than on motor adaptation), and outcome also depends on whether implicit or explicit strategies are needed.
Cognition
The most important factor that should be taken into account while studying the involvement of the cerebellum in cognition is that cerebellar stimulation usually interferes with cognitive functioning in a very subtle manner. Specific methods to measure potential effects and timing are crucial to accurately observe the impact of cerebellar stimulation. A lot of parallels can be drawn with the findings in the motor literature, with a differential impact on explicit and implicit processes depending on the nature of the task, a role for the complexity of the task, and a role of the cerebellum in perception/processing, error correction, learning, and accuracy.
Affect
Not much can be said about the impact of cerebellar stimulation on affective processing. There are too few studies to substantiate any conclusions, although there are indications from experimental and clinical studies that the cerebellum is involved in affective and somatosensory processing.19
Clinical practice
In clinical populations, TMS and tDCS seem to have a lot of potential as substituting or adjuvant therapeutic tools. It seems that not only in motor deficiencies, but also in a variety of non-motor and psychiatric conditions, cerebello-cerebral functional connectivity is disrupted, which might be restored employing cerebellar stimulation. Several isolated studies have shown that repeated sessions of cerebellar stimulation may exert a long-lasting positive effect on certain deficits. However, in order to establish TMS and tDCS as standard clinical practice techniques, it is crucial to learn more about the working mechanisms and impact of the different stimulation protocols.
Conclusion
Cerebellar TMS and tDCS are both promising and novel techniques to probe and modulate cerebellar excitability. However, there is a great need for systematic and methodological research to clarify the underlying pathophysiological mechanisms, and the specific impact of the different paradigms and parameters on cerebellar excitability/ activity as well as on remote plasticity of the motor cortex.20 In addition, more research has to be directed to the specific working mechanisms of TMS and tDCS and how these techniques might differ and be differentially used in specific settings. When applying cerebellar stimulation, it has to be kept in mind that the cerebellum has a divergent cytoarchitecture and functioning, and is connected to the cerebrum/spinal cord in different ways, which might result in a very unique response to magnetic and/or electrical stimulation that is not comparable to cerebral stimulation.
References
- Grimaldi G, Argyropoulos GP, Boehringer A, Celnik P, Edwards MJ, Ferrucci R, et al. Non-invasive Cerebellar Stimulation—a Consensus Paper. The Cerebellum 2014;13(1):121–38.
- Janssen AM, Oostendorp TF, Stegeman DF. The coil orientation dependency of the electric field induced by TMS for M1 and other brain areas. J NeuroEngineering Rehabil 2015;12(1). Available from: http://www.jneuroengrehab.com/content/12/1/47
- Sekino M, Hirata M, Sakihara K, Yorifuji S, Ueno S. Intensity and Localization of Eddy Currents in Transcranial Magnetic Stimulation to the Cerebellum. IEEE Trans Magn 2006;42(10):3575–7.
- Tomlinson SP, Davis NJ, Bracewell RM. Brain stimulation studies of non-motor cerebellar function: A systematic review. Neurosci Biobehav Rev 2013;37(5):766–89.
- Müller N, Lorenz I, Langguth B, Weisz N. rTMS Induced Tinnitus Relief Is Related to an Increase in Auditory Cortical Alpha Activity. PLoS ONE 2013;8(2):e55557.
- Priori A. Brain polarization in humans: a reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin Neurophysiol 2003;114(4):589–95.
- Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol 2016;127(2):1031–48.
- Rahman A, Reato D, Arlotti M, Gasca F, Datta A, Parra LC, et al. Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects: Somatic and terminal origin of DCS effects. J Physiol 2013;591(10):2563–78.
- Deans JK, Powell AD, Jefferys JGR. Sensitivity of coherent oscillations in rat hippocampus to AC electric fields. J Physiol 2007;583(Pt 2):555–65.
- Rahman A, Toshev PK, Bikson M. Polarizing cerebellar neurons with transcranial Direct Current Stimulation. Clin Neurophysiol 2014;125(3):435–8.
- Hardwick RM, Lesage E, Miall RC. Cerebellar Transcranial Magnetic Stimulation: The Role of Coil Geometry and Tissue Depth. Brain Stimulat 2014;7(5):643–9.
- Manto M, Hampe CS, Rogemond V, Honnorat J. Respective implications of glutamate decarboxylase antibodies in stiff person syndrome and cerebellar ataxia. Orphanet J Rare Dis 2011;6(3).
- Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, Imamizu H, et al. Consensus Paper: The Cerebellum’s Role in Movement and Cognition. The Cerebellum 2014;13(1):151–77.
- Meola A, Comert A, Yeh F-C, Sivakanthan S, Fernandez-Miranda JC. The nondecussating pathway of the dentatorubrothalamic tract in humans: human connectome-based tracto- graphic study and microdissection validation. J Neurosurg 2016;124(5):1406–12.
- Bastian AJ, Mink JW, Kaufman BA, Thach WT. Posterior vermal split syndrome. Ann Neurol 1998;44(4):601–10.
- Schmahmann JD, Pandya, D DN. The cerebrocerebellar system. In: Schmahmann JD, editor. The cerebellum and cognition, International review of neurobiology. San Diego CA: Academic Press; 1997. p. 31–60.
- Mariën P, De Smet HJ, Wijgerde E, Verhoeven J, Crols R, De Deyn PP. Posterior fossa syndrome in adults: A new case and comprehensive survey of the literature. Cortex 2013;49(1):284–300.
- Manto M, Bower JM, Conforto AB, Delgado-García JM, da Guarda SNF, Gerwig M, et al. Consensus Paper: Roles of the Cerebellum in Motor Control—The Diversity of Ideas on Cerebellar Involvement in Movement. The Cerebellum 2012;11(2):457–87.
- Schutter DJ, van Honk J. The cerebellum on the rise in human emotion. The Cerebellum 2005;4(4):290–4.
- Oulad Ben Taib N, Manto M. The in vivo reduction of afferent facilitation induced by low frequency electrical stimulation of the motor cortex is antagonized by cathodal direct current stimulation of the cerebellum. Cerebellum & Ataxias 2016;3(1). Available from: http://cerebellumandataxias.biomedcentral.com/articles/10.1186/s40673-016-0053-3.



