Abstract
The fourth update of the McDonald criteria enables an earlier diagnosis of MS in people presenting with typical symptoms. It broadens MRI evidence for dissemination in space to include symptomatic and cortical lesions and allows dissemination in time to be demonstrated by unmatched oligoclonal bands in the CSF as well as by new or enhancing MRI lesions. To avoid misdiagnosis, it should be used with caution in patients with atypical symptoms or in populations in which MS is uncommon.
Key take home messages
- The diagnostic criteria for MS have been recently updated
- These criteria should only be applied to populations in whom MS is common and patients who present with typical symptoms for which no better explanation can be found
- All patients with suspected MS should have an MRI brain scan; spinal cord imaging is not mandatory
- Unmatched oligoclonal bands in the CSF can be used as a substitute for demonstrating dissemination in time, allowing an earlier diagnosis than was previously possible
The history of diagnostic criteria for multiple sclerosis (MS)
Multiple Sclerosis (MS) became widely recognised after Jean-Martin Charcot (1825 – 1893) (Figure 1a) described it in his lectures in the late 18th century. Prior to this patients with MS would typically have been described as having a palsy, paralysis or paraplegia, without much understanding as to the cause.
In May 1960, a symposium on the ‘Evaluation of Drug Therapy in Neurologic and Sensory Diseases’ held at the University of Wisconsin concluded that criteria for MS were required to provide a common ground of terminology amongst investigators to facilitate therapeutic trials.
A committee of experts chaired by George Schumacher (1912 – 2008) was formed and published the Schumacher criteria in 1965.1 This enabled a diagnosis of definite MS in:
- a typically aged individual (defined as between 10 and 50 years)
- with a compatible history – attacks lasting at least 24 hours, separated by at least a month; or in the case of progressive MS, a slow or step-wise progression of disability over a period of at least six months
- and objective clinical evidence of lesions in two or more distinct sites in the white matter of the central nervous system
- with no more satisfactory explanation.
The Schumacher criteria were purely clinical, although investigations were encouraged (blood, urine, chest X-ray, CSF analysis) to exclude alternative conditions.
Over the next few years modifications to the Schumacher criteria were published2,3 but in 1983 the Schumacher criteria were replaced by criteria developed by a committee chaired by Charles Poser (1923 – 2010).4 The Poser criteria incorporated laboratory and clinical tests developed in the previous decade to support the diagnosis with ‘paraclinical evidence’ of lesions. This included evoked potentials, computed tomography (CT) or NMR scans (as MRI was known in its early days), as well as induced hyperthermia (the hot bath test) and expert urological assessment. The acceptable age of onset was extended to 10 to 59 years of age. The criteria emphasised that symptoms should be consistent with MS and the diagnosis made by a ‘competent neurologist’.
The criteria divided patients into two groups – ‘Definite’ and ‘Probable’ MS – each with two sub-groups – ‘Clinical’ and ‘Laboratory-supported’; the latter referring to the presence of oligoclonal bands or raised IgG in the CSF. Clinically Definite MS became the requirement for entry into therapeutic trials of the time and required two attacks and objective clinical evidence of two lesions; or two attacks with objective evidence of one lesion and paraclinical evidence of another separate lesion. Certain historical symptoms could be substituted for clinical evidence in some instances, e.g. Lhermitte’s phenomenon in the absence of cervical spondylosis; painful optic neuritis in an under 50-year-old, trigeminal neuralgia in an under 40-year-old.
In 2001, Poser’s criteria were replaced by McDonald’s criteria,5 developed by the International Panel on MS Diagnostics, chaired at their first meeting by Ian McDonald (1933-006) (Figure 1b, below right).
These criteria placed a much greater emphasis on the use of MRI lesions (areas of T2 hyperintensity at least 3mm in cross-section) to demonstrate dissemination of disease in space and time. These criteria enabled a diagnosis of MS after a single clinical attack which allowed clinical trials to include patients at a much earlier stage than had previously been possible. Progressive MS, which had not been addressed by the Poser criteria, was defined in McDonald’s criteria and required the presence of oligoclonal bands or raised IgG index in the CSF supported by typical MRI findings +/- delayed visual evoked potentials (VEPs) and dissemination in time demonstrated either by progression of disability over a year or new MRI lesions.
The McDonald panel have subsequently met approximately every 5-years to refine and simplify the criteria.6,7 The number of lesions required to provide evidence of dissemination in space have been reduced and evidence of dissemination of disease in time may be obtained from a single MRI with both enhancing and non-enhancing lesions. The criteria for progressive MS were changed so that insidious neurological progression became the main requirement and oligoclonal bands or elevated IgG index in the CSF were no longer mandatory if there were typical MRI findings in the brain and spinal cord. The term ‘possible MS’ was added for people with a typical clinically isolated syndrome who did not meet the criteria.
The 2017 revisions to the McDonald criteria
Following meetings in November 2016 and May 2017 the fourth version of the McDonald criteria was published in Lancet Neurology in January 2018.8 This panel was expanded to include additional expertise in clinical, imaging and laboratory aspects of MS diagnosis, and to address criticisms that they were only applicable to European and North American populations, included a broader representation from different geographical regions. Changes to the diagnostic criteria were evidence-based and not just based on expert opinion.
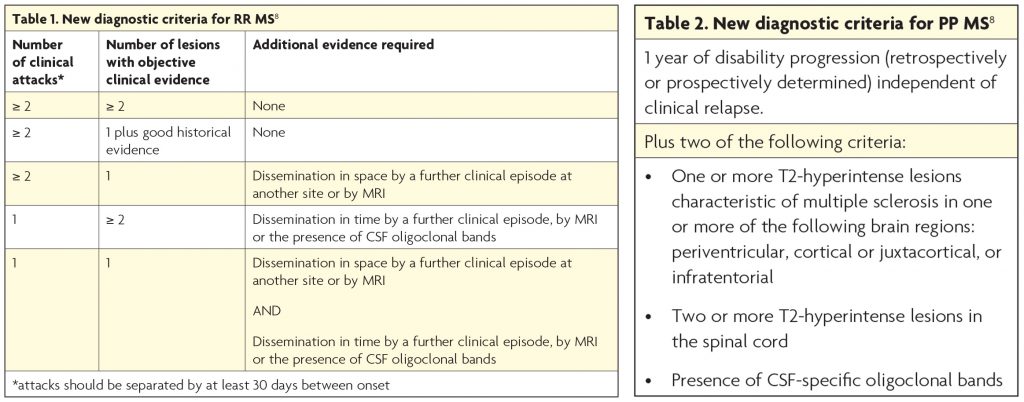
The most significant change is the revitalisation of the role of CSF analysis. The finding of two or more oligoclonal bands was found to be more reliable than a raised IgG index and their presence has been shown to have high predictive value for conversion from clinically isolated syndromes to MS. The panel agreed the presence of unmatched oligoclonal bands in the CSF could confirm dissemination in time in place of clinical or MRI evidence. The panel also stressed the importance of CSF in excluding MS mimics.
The MRI criteria for dissemination in space have also been changed. Cortical lesions can now be counted in place of juxtacortical ones when assessing for lesions in one of the four typical sites (the others sites are periventricular, brainstem or spinal cord lesion). However, cortical lesions are not well appreciated on currently available clinical MRI scans conducted outside of research and so this will not have a great impact on most clinicians. The panel did not increase the number of periventricular lesions required, as was recommended in a 2016 MAGNIMS MRI criteria paper,9 but suggested this may be advisable in older patients and those at high risk of having white matter lesions, e.g. vascular risk factors, migraine. They did allow for the inclusion of symptomatic lesions when assessing for radiological evidence of dissemination in space – an exception being high signal in the optic nerves in people with optic neuritis.
As with the criteria that have predated it, the McDonald criteria do enable MS to be diagnosed without the need for any supporting investigations, but the panel recommended all patients in whom a diagnosis of MS was being considered should have an MRI brain. An MRI of the cord is not mandatory but advisable when signs localise to the cord, in progressive MS and in populations in whom MS is more unusual.
Whilst the sensitivity and specificity of the McDonald MRI criteria have been shown to be high when applied to patients with typically clinically isolated syndromes suggestive of MS,10 there have been a number of papers published demonstrating that they over-diagnose MS in a ‘real world’ setting.11-13 The McDonald criteria were not developed to diagnose MS in patients with atypical symptoms or to distinguish it from other conditions which can cause high signal in the white matter lesions on MRI, e.g. acute disseminated encephalitis (ADEM), neuromyelitis optica spectrum disorders (NMOSD), vascular disease, migraine and even normal ageing. Caution should be exercised in people outside the typical presenting age for MS (although MS can present in childhood and in individuals over 60 years) and in ethnic groups in whom MS is uncommon. The benefits of expert neuro-radiological input cannot be over-emphasised.
A diagnosis of multiple sclerosis should never be made based on MRI appearances alone, although some patients with these so-called Radiologically Isolated Syndromes will develop typical symptoms of MS in time14 and may require counselling and follow-up.
Application of the 2017 revised McDonald criteria in patients with typical clinically isolated syndromes followed up for five years has been demonstrated to have greater sensitivity but less specificity for a second attack than the 2010 criteria with similar accuracy. 15 This means it will diagnose more patients with less active MS.
In conclusion, the McDonald Criteria are helpful in providing an accurate and earlier diagnosis of MS in patients following a single attack with typical symptoms, however when the presentation is atypical, or in populations in which MS is uncommon, investigation should be extended beyond MRI, with a low threshold for further investigation, particularly examination of the CSF.
References
- Schumacher GA, Beebe G, Kibler RF, Kurland LT, Kurtzke JF, Mcdowell F, et al. Problems Of Experimental Trials Of Therapy In Multiple Sclerosis: Report By The Panel On The Evaluation Of Experimental Trials Of Therapy In Multiple Sclerosis. Ann N Y Acad Sci. 1965 Mar 31;122:552–68.
- Rose AS, Ellison GW, Myers LW, TOURTELLOTTE WW. Criteria for the clinical diagnosis of multiple sclerosis. Neurology. 1976 Jun;26(6 PT 2):20–2.
- McDonald WI, HALLIDAY AM. Diagnosis and classification of multiple sclerosis. Br Med Bull. 1977 Jan;33(1):4–9.
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. Wiley Subscription Services, Inc., A Wiley Company; 1983 Mar;13(3):227–31.
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung H-P, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Vol. 50, Annals of neurology. 2001. pp. 121–7.
- Polman CH, Reingold S, Edan G, Filippi M, Hartung H-P, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. Wiley Subscription Services, Inc., A Wiley Company; 2005 Dec;58(6):840–6.
- Polman CH, Reingold S, Banwell B, Clanet M, Cohen J, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011 Feb;69(2):292–302.
- Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2017 Dec 21;17(2):162–73.
- Filippi M, Rocca MA, Ciccarelli O, De Stefano N, Evangelou N, Kappos L, et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. Elsevier; 2016 Jan 25;0(0):292–303.
- Brownlee WJ, Swanton JK, Altmann DR, Ciccarelli O, Miller DH. Earlier and more frequent diagnosis of multiple sclerosis using the McDonald criteria. J Neurol Neurosurg Psychiatr. BMJ Publishing Group Ltd; 2015 May;86(5):584–5.
- Whiting P, Harbord R, Main C, Deeks JJ, Filippini G, Egger M, et al. Accuracy of magnetic resonance imaging for the diagnosis of multiple sclerosis: systematic review. BMJ. British Medical Journal Publishing Group; 2006 Apr 15;332(7546):875–84.
- Solomon AJ, Bourdette DN, Cross AH, Applebee A, Skidd PM, Howard DB, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: A multicenter study. Neurology. American Academy of Neurology; 2016 Sep 27;87(13):1393–9.
- Rosenkranz SC, Kaulen B, Neuhaus A, Siemonsen S, Köpke S, Daumer M, et al. Low clinical conversion rate in clinically isolated syndrome patients – diagnostic benefit of McDonald 2010 criteria? Eur J Neurol. 2018 Feb;25(2):247–9.
- Okuda DT, Mowry EM, Beheshtian A, Waubant E, Baranzini SE, Goodin DS, et al. Incidental MRI anomalies suggestive of multiple sclerosis: the radiologically isolated syndrome. Neurology. 2009 Mar 3;72(9):800–5.
- Van der Vuurst de Vries RM, Mescheriakova JY, Wong YYM et al. Application of the 2017 Revised McDonald Criteria for Multiple Sclerosis to Patients With a Typical Clinically Isolated Syndrome. JAMA Neurology 2018; doi:10.1001/jamaneurol.2018.2160

