Abstract
Dengue produces neurological manifestations uncommonly and acute cerebellar ataxia (ACA) is particularly rare. We describe a traveller returning to a non-endemic area who presented with rash, abdominal pain, fever and classical laboratory findings consistent with dengue fever who then developed ACA. MRI scan showed focal nodular leptomeningeal enhancement over the cortex. Other potential causes were excluded. Symptoms largely resolved within one month with supportive care. This case records a rare complication in a traveller returning to a non-endemic area and demonstrates neuroimaging abnormalities. It is important to include dengue virus infection in the differential diagnosis of ACA in returned travellers.
Learning points
- Acute cerebellar ataxia (ACA) is a rare complication of dengue
- Neuroimaging findings in dengue are variable and can include meningeal enhancement
- Consider dengue as a cause of ACA in returned travellers from endemic areas
Introduction
Dengue is a mosquito-borne arboviral infection that causes multi-systemic disease with considerable morbidity and mortality.1 Neurological sequelae of dengue virus infection are uncommon and acute cerebellar ataxia (ACA) is particularly rare.2 This infectious aetiology is an important part of the differential diagnosis of ACA in travellers returning from endemic areas.
Case report
A 73-year-old Caucasian woman presented with right-sided abdominal pain, diarrhoea and nausea. She had recently returned from Fiji. She was systemically well during her trip and had no relevant exposures or obvious bites. On return home, she was fatigued and gradually developed abdominal pain, nausea, watery diarrhoea, subjective fevers and rigors, generalised arthralgias and myalgias. She had no urinary symptoms, rashes or pruritus. Three days after her return she became unsteady on her feet with associated headache.
Her past history was significant for diverticular colitis treated with mesalazine, appendicectomy, hypertension, hiatus hernia, gastro-oesophageal reflux disease, osteoarthritis with left total knee replacement, depression, previous caesarean section and tubal ligation, dyslipidaemia and drop attacks of uncertain aetiology. She had a loop recorder in situ for investigation of potential arrhythmia. Her medications included fluoxetine, ranitidine, pantoprazole, rosuvastatin, mesalazine, aspirin, celecoxib, fexofenadine, glucosamine, irbesartan, hydrochlorothiazide, paracetamol and vitamin supplements.
On examination, she was alert, orientated and haemodynamically stable with a low grade fever to 37.7⁰C. Her abdomen was soft and generally tender without peritonitis. Bowel sounds were normal. Eye movements and vestibulo-ocular reflexes were normal. There was no nystagmus. Speech was normal. She had mild proximal weakness, easily elicited deep tendon reflexes and flexor plantar responses. There was a slight terminal tremor on the left with mild dysdiadochokinesis but no dysmetria. There were no extrapyramidal signs. Gait was wide-based and ataxic. Tandem gait could not be attempted. Romberg’s was negative. Sensory examination was normal, including proprioception. There was a subtle blanching maculopapular rash over the trunk.
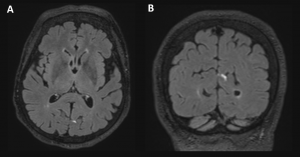
Full blood count at nadir showed haemoglobin 110 g/L, leukocytes 1.8×109/L, neutrophils 0.8×109/L, lymphocytes 0.6×109/L and platelets 82×109/L. Electrolytes, urea and creatinine were normal. There was mild liver enzyme derangement (ALT 24 U/L, AST 43 U/L, ALP 76 U/L, GGT 37 U/L), low albumin (27 g/L) and elevated lactate dehydrogenase (259 U/L). Vitamin B12, folate and iron were replete. Haemolytic and disseminated intravascular coagulation screens were negative. Coagulation profile was normal. C reactive protein was raised to 20.8 mg/L. Blood and stool cultures were negative. Dengue virus NS1 antigen and IgM antibodies were positive. Respiratory multiplex PCR and malaria testing were negative. Cytomegalovirus serology indicated previous infection. Antineuronal antibody panel was negative. MRI brain scan showed two focal areas of nodular leptomeningeal enhancement in the left occipital region as well as over the left frontal cortex (Figure 1).
The patient received supportive care. Her symptoms had largely resolved at follow up, one month after initial presentation.
Discussion
We consider this to be a case of ACA due to dengue fever with warning signs.3 This represents an intermediate category of dengue severity that requires strict monitoring and supportive care.3 Other potential causes of ACA have been excluded and the syndrome occurred concurrently with typical clinical and laboratory features of dengue virus infection.1 This case is important because it records this rare complication in a traveller returning to a non-endemic area and demonstrates neuroimaging abnormalities.
Neurological manifestations of dengue are uncommon and include encephalopathy, encephalomyelitis, immune-mediated phenomena, neuro-ophthalmic and neuromuscular disorders.1 Cerebellar ataxia has been reported in endemic areas.4
Neuroimaging and serological testing in this case show no evidence of a vascular, neoplastic, paraneoplastic, demyelinating or toxic cause. CSF examination was not performed, however the clinical (rash, abdominal pain, fever) and laboratory (thrombocytopenia, liver enzyme derangement, positive serology) features favour the diagnosis of dengue-related ACA.
Dengue virus causes neurological sequelae via direct viral neurotropism, delayed post-infectious immunological mechanisms and systemic metabolic derangements.2,3,5 The former pathogenic mechanism is most likely in this case given the early development of ACA in the setting of acute dengue virus infection. Autopsy studies demonstrating dengue antigens in cerebellar tissue offer further support for this mechanism.5
Imaging findings in dengue virus infection are variable and can include meningeal enhancement, as seen in this case6 (Figure 1). The location of these changes do not correspond to the clinical picture of ACA and may refect a more generalised meningeal inflammatory response to the virus. The absence of cerebellar changes is not uncommon.4
Prognosis is generally favourable,2,4 as seen in this case, although dengue virus infection can be fatal.1
This case highlights the importance of including dengue virus infection in the differential diagnosis of ACA in travellers returning from endemic areas.
References
- Carod-Artal FJ et al. Neurological complications of dengue virus infection. Lancet Neurol. 2013;12(9):906-919. https://doi.org/10.1016/S1474-4422(13)70150-9
- Weeratunga PN et al. Neurological manifestations of dengue: a cross sectional study. Travel Med Infect Dis. 2014;12(2):189-93. https://doi.org/10.1016/j.tmaid.2013.11.001
- World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. 2009, Geneva
- Weeratunga PN et al. Spontaneously resolving cerebellar syndrome as a sequelae of dengue viral infection: a case series from Sri Lanka. Pract Neurol. 2014;14(3):176-8. https://doi.org/10.1136/practneurol-2013-000571
- Ramos C et al. Dengue virus in the brain of a fatal case of hemorrhagic dengue fever. J Neurovirol. 1998;4(4):465-8. https://doi.org/10.3109/13550289809114548
- Bhoi SK et al. Cranial imaging findings in dengue virus infection. J Neurol Sci. 2014;342(1-2):36-41. https://doi.org/10.1016/j.jns.2014.04.018


