Abstract
Cerebrospinal fluid (CSF) hydrothorax has been reported most commonly as a complication of ventriculo-pleural/-peritoneal shunt insertion, but also due to duro-pleural fistula. Here we report a case of CSF hydrothorax in a rehabilitation patient due to duro-pleural fistula following thoracotomy for thoracic myelopathy secondary to disc protrusion. This case highlights the need for high clinical suspicion following thoracotomy, and urgent specialist input from the neurosurgical team.
Case Report
A 58-year-old male with a history of chronic back pain suffered severe pain to his mid thoracic back after heavy lifting. After 10 days his symptoms progressed to include bilateral lower limb weakness, neuropathic pain in his right foot, and paraesthesia around his umbilicus. He also reported that his bowels had been more ‘sluggish’ and he had difficulty passing urine. He presented to his local A&E, and was found to have T7/8 disc protrusion with cord compression and early myelopathy, in addition to L4/5 severe spinal canal stenosis and generalised degenerative changes.
The patient was transferred to the regional neurosurgical specialist centre and underwent right sided anterior thoracotomy for resection of T7/8 disc protrusion. Trench vertebrectomy was performed. A large area of calcified ossification of the posterior longitudinal ligament (OPLL) was found, with adherent dura at the level of calcification. OPLL was dissected and resected into the vertebrectomy defect and the cord was decompressed. As the OPLL was adherent to the dura, this dissection resulted in dural tear. Rib was used for vertebrectomy fusion. The dura was reconstructed with dural patch and tisseel glue, and a right anterolateral plate and screws were used for fixation from T6-T9. Further surgical intervention was planned in future for the lumbosacral canal stenosis. Post-operatively he developed headache and neck ache felt to be due to cerebrospinal fluid (CSF) leak, and had bilateral lower limb weakness in keeping with spinal cord injury. CT brain confirmed collapse of the ventricular system, but the patient was neurologically stable and no further intervention was felt to be indicated by the neurosurgical team.
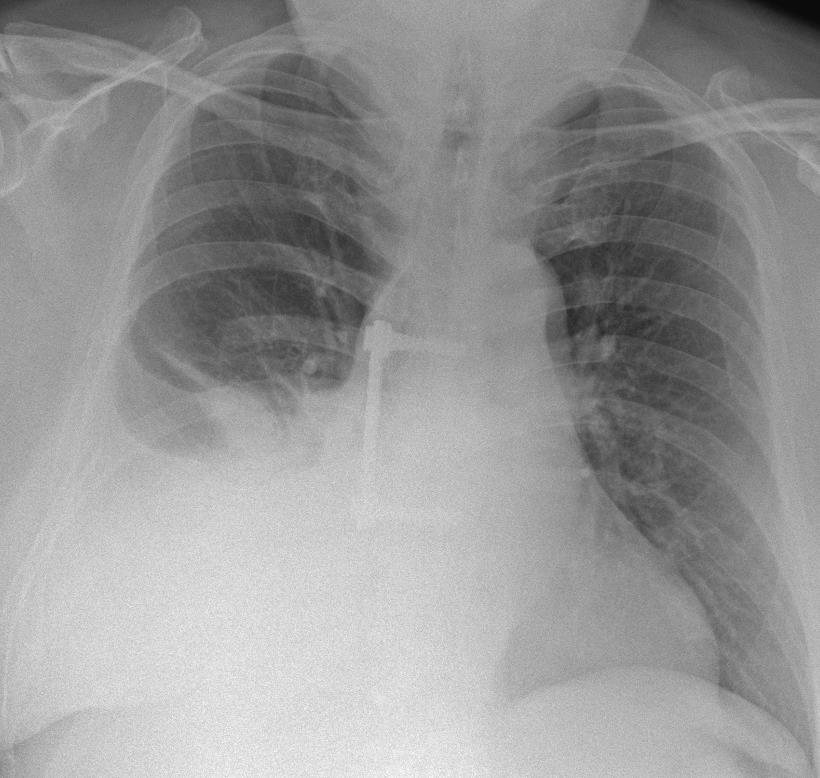
Once stable post-operatively, he was transferred to the regional spinal cord injury centre for ongoing rehabilitation. On admission it was noted that his headache was still ongoing since the operation, and after 4 days he became unwell with suspected chest infection. Examination revealed reduced air entry in the right lower lobe, and chest X-ray (CXR) showed patchy shadowing in that region. He completed a course of antibiotics, then after a further 2 weeks he deteriorated again with shortness of breath on exertion. Chest X-ray demonstrated a moderate right-sided pleural effusion, and this was confirmed by CT. He was reviewed by the respiratory team, and a therapeutic aspiration was performed. 1900mls of light straw-coloured fluid was obtained and sent for biochemistry, culture and sensitivity, and cytology. Biochemistry showed a borderline exudate, but otherwise investigations were unremarkable. A CXR after the aspiration showed a moderate pleural effusion still remained, so a repeat therapeutic aspiration was performed 1 week after the first. 2000mls of straw-coloured fluid was aspirated, and investigations showed a similar picture as previously. The team was concerned that he had no obvious reason for a typical cause of effusion – there were no signs of heart failure and B-type natriuretic peptide was normal, there was no evidence of a renal or liver cause, and no evidence of an underlying cause for exudative effusion. Due to the intra-operative dural tear, suspicion of CSF leak post-operatively, and ongoing headache, we felt that the effusion could be CSF. We were unsure whether this could result in such a large effusion without causing more significant neurological symptoms. We contacted the laboratory to test his CSF for beta-trace protein (BTP); the biochemist was initially reluctant due to the chance of a false negative when performing the test on pleural fluid, but agreed because there was a high clinical suspicion. This was reported as showing BTP 15.6mg/L, suggesting the fluid was in fact likely CSF.
We contacted the neurosurgical team who arranged urgent transfer back under their care. MRI imaging showed kinking and high signal within the cord at the level of the recent surgery, with the cord being anteriorly positioned within the thecal sac. Lumbar drain insertion was initially difficult due to the patient’s degenerative spinal disease, so lumbar decompression was performed, and a drain inserted under direct vision. The patient then developed signs of re-accumulation of fluid, and CXR confirmed a larger pleural effusion. He was transferred to intensive care for chest drain insertion, and 2000mls was drained. Initially the plan was to monitor with a lumbar drain in-situ to allow time for spontaneous closure of the fistula. However, due to ongoing fluid accumulation despite conservative measures, he was taken to theatre for definitive surgical closure. Following successful treatment, he returned to our care to continue rehabilitation.
Discussion
Patients with thoracic disc herniation often present late as thoracic nerves have no limb motor control. Thoracic radiculopathy typically presents with neuropathic pain across the chest, which can be misdiagnosed as shingles or pain of a muscular/cardiac/abdominal cause. In more advanced stages of disc herniation myelopathy can develop. The majority of patients with thoracic myelopathy simply have back pain alone prior to developing ataxia and lower limb weakness, discoordination and sensory deficit.
The choroid plexus produces around 400 – 600mls of CSF per day, whilst the healthy pleural cavity can reabsorb up to around 200mls of fluid per day [1]. If there is a significant CSF leak into the pleural space, an effusion can develop relatively quickly. Inflammation can decrease the ability of the pleural space to reabsorb fluid, and further expedite the accumulation of fluid.
Routine pleural fluid investigations tend to show a clear/straw coloured transudate, but are not diagnostic for CSF. BTP is made in the choroid plexus within the ventricles of the brain, and is found in the central nervous system in much higher concentrations than elsewhere in the body. When at diagnostic levels, BTP is highly specific and sensitive for the diagnosis of CSF. When testing pleural fluid there is a risk of CSF being diluted by the presence of other fluid, thus giving a false negative result. BTP is generally tested for when there is a high suspicion of CSF leak, rather than as part of routine investigations. Similarly, beta-2-transferrin can also be used for diagnosis.
Most reported cases of CSF hydrothorax are secondary to ventriculo-pleural/-peritoneal shunt complications [2-5]. Other reports describe duro-pleural fistula as seen in this case, most commonly when both the dural and pleural membranes are disrupted [1,6,7]. Clinicians should have a high index of suspicion with effusions following thoracic spinal surgery, where anterolateral thoracotomy is the standard approach [7]. Negative pressure within the pleural space causes a pressure gradient, allowing fluid to pass into the pleural cavity. Draining can cause the pressure gradient to increase, resulting in more CSF entering the pleural cavity [1,7]. This can lower the CNS pressure and put the patient at risk of developing intracranial hypotension that can lead to subdural hygromas, subdural haemorrhage or brain herniation [1]. Furthermore, chest drain insertion poses a significant risk of CNS infection [7]. Spontaneous resolution is rare, and in most cases surgical closure of the fistula is required [6,8]. In conclusion, high clinical suspicion of CSF hydrothorax in these patients should be prompted by the surgical approach, and history of post operative postural headache, dyspnoea, cough or respiratory distress. A thorough respiratory clinical evaluation is necessary, along with CXR and specialised tests such as beta 2 transferrin. Neurosurgical input for specialist advice should be sought without delay.
Key Points:
- Thoracic disc prolapse may present as radiculopathy typically with neuropathic pain across the chest, but motor deficit can be seen in more advanced stages when myelopathy develops secondary to cord compression.
- The choroid plexus produces 400-600mls of cerebrospinal fluid per day whilst the healthy pleural cavity can reabsorb around 200mls per day, meaning a CSF effusion can develop relatively quickly.
- Testing for beta-trace protein is highly specific and sensitive in detecting CSF.
- Draining a CSF effusion can increase the pressure gradient, allowing more fluid to enter the pleural cavity, and risking brain herniation.
References
- Kierstead P, Lanks C. A 26-Year-Old Man With a Pleural Effusion and Headache. Chest. 2018 Oct;154(4):e113-e117. https://doi.org/10.1016/j.chest.2018.04.011
- Ulus A, Kuruoglu E, Ozdemir SM, Yapici O, Sensoy G, Yarar E, et al. CSF hydrothorax: Neither migration of peritoneal catheter into the chest nor ascites. Case report and review of the literature. Childs Nerv Syst. 2012;28:1843-8. https://doi.org/10.1007/s00381-012-1862-1
- Kim JH, Roberts DW, Bauer DF. CSF hydrothorax without intrathoracic catheter migration in children with ventriculoperitoneal shunt. Surg Neurol Int. 2015;6(Suppl 11):S330-3. https://doi.org/10.4103/2152-7806.161408
- Cato-Addison WB, Strachan R. CSF hydrothorax: An unusual cause of pleural effusion. Br J Neurosurg. 2015;29(4):600-2. doi: 10.3109/02688697.2015.1019420. https://doi.org/10.3109/02688697.2015.1019420
- Wong E, Jeganathan V, Wreghitt S, Davis G, Wimaleswaran H, Howard ME. Worsening respiratory failure in an adult hydrocephalic patient with a ventriculo-pleural shunt. Respirol Case Rep. 2020 Sep 26;8(8):e00660. https://doi.org/10.1002/rcr2.660
- Saini P, Callejas L, Gudi M, Grosu HB. Recurrent pleural effusion due to duropleural fistula. J Bronchology Interv Pulmonol. 2014 Jul;21(3):265-6. https://doi.org/10.1097/LBR.0000000000000067
- Maskin LP, Raimondi A, Hlavnicka A, Díaz MF, Roura N, Wainsztein N. Duropleural fistula revealed by neurological manifestations: an unusual cause of pleural effusion. Anaesth Intensive Care. 2010 Jan;38(1):201-3. https://doi.org/10.1177/0310057X1003800132
- Lin JP, Zhang SD, He FF, Liu MJ, Ma XX. The status of diagnosis and treatment to intracranial hypotension, including SIH. J Headache Pain, 18: 4, 2017. https://doi.org/10.1186/s10194-016-0708-8





