Abstract
We describe an unusual case of stroke manifesting as alien hand syndrome (AHS) causing sudden onset of abnormal hand movements. The patient reported his left arm would move outside his control and grab things in his surroundings without his conscious will. He felt his arm did not belong to him. Examination showed left arm brisk reflexes, astereognosis, and agraphesthesia in the left hand. Imaging revealed an established right parietal lobe ischaemic stroke. Lesions of the parietal cortex can cause AHS by disrupting the interpretation of somatosensory feedback when a movement is made and decreasing the ability to consciously monitor motor intentions. A low threshold should be adopted for arranging brain imaging and a thorough neurological examination is needed in any sudden onset movement disorder.
Alien hand syndrome (AHS) was first described in 1908 as a motor apraxia [1]. The term ‘alien hand’ originates from the French ‘main étrangère’ which Brion et al. used to describe the foreignness of the limb in patients with corpus callosum lesions [2]. AHS is defined by two criteria: the hand moves involuntarily and acts as though it has its own will [3]. Here, we report a case of AHS caused by a pure parietal stroke involving the postcentral gyrus.
Case presentation
A 70-year-old right-handed man presented to the movement disorders clinic reporting that his left arm was ‘jumping all over the place’. His symptoms started a month earlier, when his arm movements suddenly woke him up from sleep, touching him and moving spontaneously outside his control. His arm would shoot upwards, needing to be controlled by his right hand. It would grab things in his surroundings without his conscious will, and he felt it did not belong to him. This caused difficulties with tasks requiring both hands, such as buttoning his shirt. His left arm was not weak, but he was unable to maintain its posture and struggled to hold a cup. The other limbs, face, vision, and speech were not affected. His past medical history included hypertension and hyperlipidaemia, and medications included aspirin, atorvastatin, lansoprazole, and naproxen.
On examination, his cranial nerves were intact apart from a mild left facial droop. Power and sensation to pinprick, fine touch, and vibration sensation were normal in all four limbs. However, he had astereognosis and agraphesthesia in the left hand, suggesting cortical sensory loss. He had mild left dysdiadochokinesia and bilateral intention tremor, but no bradykinetic rigidity. Reflexes were brisk in the left arm and plantar responses were flexor bilaterally. There was no ataxia.
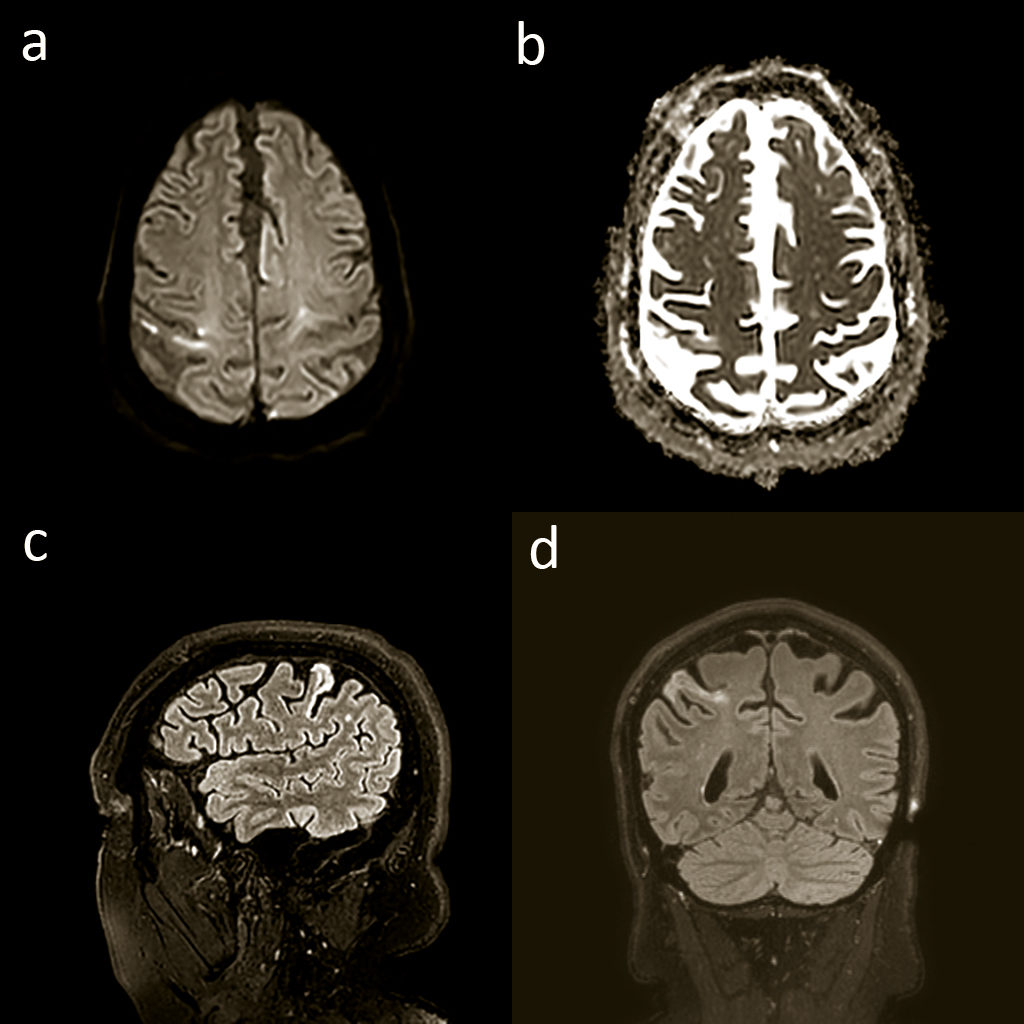
His MRI brain showed an established ischaemic stroke in the right postcentral gyrus of the parietal lobe, best seen on the fluid attenuation inversion recovery images (Figure 1), which was the correct age for when his symptoms started.
At follow-up after six weeks, the patient reported that the involuntary movements of his left arm reduced dramatically to become very infrequent, which do not affect him significantly. He no longer reports the feeling of foreignness.
Discussion
In a literature review by Scepkowski et al, frontal, callosal, and posterior subtypes were used to describe AHS [4]. The frontal type afflicts the dominant hand, resulting from left medial frontal and callosal damage. Patients develop a grasp reflex and compulsive tool manipulation. Damage to the corpus callosum with or without bilateral or right frontal lesions causes the callosal type, affecting the non-dominant hand. In this type, patients develop inter manual conflict, where one hand acts at cross-purposes with the other. Posterior AHS is less commonly described and is caused by injury to the thalamus and parietal, occipital, and temporal lobes. It causes uncoordinated purposeless hand movements, arm levitation, and a feeling of foreignness.
Several neurological conditions can cause AHS other than stroke, including brain tumours, corpus callosum surgery, aneurysms, or degenerative disease. Corticobasal degeneration (CBD) is a rare neurodegenerative disease involving the cerebral cortex and the basal ganglia. It can cause AHS, but additionally causes parkinsonism, apraxia, aphasia, and dementia [5]. This diagnosis was ruled out as it is also characterised by an insidious onset and progressive course. Our patient’s symptoms were of sudden onset and got progressively better. Also, examination did not reveal any parkinsonism and the patient had normal cognition. In addition, the MRI did not display the classical pattern of atrophy seen with CBD, although certain cases of CBD may show global atrophy alone and not a specific pattern [6].
The patient does not have a history of neurosurgery and the brain MRI scans did not show any evidence of masses or aneurysms, which ruled out vascular malformations as a cause.
Another differential diagnosis was cerebral amyloid angiopathy (CAA) which is a common age-related cerebral small vessel disease characterised by the progressive deposition of β-amyloid in the wall of cortical and leptomeningeal small arteries [7]. It can cause transient focal neurological episodes including limb jerking [8]. This can resemble the involuntary arm movements of AHS. CAA is also a common cause of lobar intracerebral haemorrhage (ICH) [9]. CAA was important to be excluded as antiplatelet therapy is a risk factor for ICH. Although the MRI changes for CAA and ischaemic stroke can be similar, no evidence was found of bleed or microhaemorrhage on blood-sensitive MRI, excluding CAA as a cause. The permanency of the symptoms also goes against a CAA diagnosis.
A literature review by the authors revealed 12 cases of AHS caused by isolated parietal lobe lesions. However, none of the cases exhibited all four features of involuntary hand movements, arm levitation, feeling of foreignness, and cortical sensory loss present in our case. We also demonstrate that an infarct limited to the postcentral gyrus can cause AHS.
Previous work using functional MRI has shown that AHS caused by parietal stroke was associated with isolated activation of the contralateral primary motor cortex, as opposed to the orchestrated activation of neural networks when a voluntary movement is made [10]. The premotor cortex generates an internal copy of a movement which is relayed to the somatosensory cortex of the parietal lobe. The somatosensory feedback generated by a movement is compared to this internal copy. If they correlate, the movement is interpreted as self-generated rather than due to an external force. In AHS, there is a deficit in creating this internal model, causing the individual to mistakenly interpret a self-generated movement as one produced by an external force [11].
Our patient was able to grasp an object in his left hand; however, he would drop it soon after, indicating a failure to maintain the action possibly due to the inability to consciously monitor his motor intention. Sirigu et al. showed that the parietal lobe plays a critical role in this function and suggests that parietal lobe stroke can cause AHS by disrupting cortico-cortical sensorimotor processing loops responsible for the awareness of the motor movement [12].
Key points
- AHS is a rare stroke chameleon and can be caused by cortical parietal stroke.
- A thorough neurological examination may reveal additional deficits such as sensory changes, visual field defects, and brisk reflexes related to stroke.
- A low threshold should be adopted for arranging brain imaging when patients present with movement disorders in the acute setting.
References
- Goldstein K. Zur Lehre der motorischen apraxie. J Psychol Neurol 1908;11:169-87.
- Brion S, Jedynak CP. Disorders of interhemispheric transfer (callosal disonnection). 3 cases of tumor of the corpus callosum. The strange hand sign. Rev Neurol (Paris) 1972;126(4):257-66.
- Gheewala G, Gadhia R, Surani SR, et al. Posterior Alien Hand Syndrome from Acute Ischemic Left Parietal Lobe Infarction. Cureus 2019;11(10):e5828. https://doi.org/10.7759/cureus.5828
- Scepkowski LA, Cronin-Golomb A. The alien hand: cases, categorizations, and anatomical correlates. Behav Cogn Neurosci Rev 2003;2(4):261-77. https://doi.org/10.1177/1534582303260119
- Ali F, Josephs KA. Corticobasal degeneration: key emerging issues. J Neurol 2018;265(2):439-45. [published Online First: 2017/10/25] https://doi.org/10.1007/s00415-017-8644-3
- Erkkinen MG, Kim MO, Geschwind MD. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb Perspect Biol 2018;10 [published Online First: 2017/07/19] https://doi.org/10.1101/cshperspect.a033118
- Coates R, Bell SM, Coley S, et al. Cerebral amyloid angiopathy: amyloid spells and cortical superficial siderosis. Pract Neurol 2015;15(2):124-6. [published Online First: 2014/11/02] https://doi.org/10.1136/practneurol-2014-000952
- Charidimou A, Peeters A, Fox Z, et al. Spectrum of Transient Focal Neurological Episodes in Cerebral Amyloid Angiopathy. Stroke 2012;43(9):2324-30. https://doi.org/10.1161/STROKEAHA.112.657759
- Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. Journal of Neurology, Neurosurgery & Psychiatry 2012;83(2):124-37. https://doi.org/10.1136/jnnp-2011-301308
- Assal F, Schwartz S, Vuilleumier P. Moving with or without will: functional neural correlates of alien hand syndrome. Ann Neurol 2007;62(3):301-06. https://doi.org/10.1002/ana.21173
- Nowak DA, Engel A, Leutbecher M, et al. Alien limb phenomenon following posterior cerebral artery stroke: a distinct clinical entity. J Neurol 2020;267(1):95-99. https://doi.org/10.1007/s00415-019-09543-2
- Sirigu A, Daprati E, Ciancia S, et al. Altered awareness of voluntary action after damage to the parietal cortex. Nat Neurosci 2004;7(1):80-84. https://doi.org/10.1038/nn1160




