Abstract
Balínt Syndrome is an acquired disorder manifesting in the inability to recognise several objects at once (simultagnosia), inaccurate visually guided limb movements despite intact motor function (optic ataxia) and the inability to make accurate voluntary saccades to visual targets despite demonstrating unrestricted range of eye movements (ocular motor apraxia). Here, we report the first case of a patient presenting with Balínt Syndrome caused by a platelet-derived growth factor receptor A mutation (PDGFRA)-induced Hypereosinophilic Syndrome (HES).
Key Points
- Balínt Syndrome can be caused by watershed infarcts, secondary to Hypereosinophilic Syndrome.
- Hypereosinophilic Syndrome can give rise to micro-emboli formation at the site of eosinophil degranulation-induced endothelial necrosis which is not necessarily detectable by cardiac MRI and/or ultrasonography.
- A mutational deletion on chromosome 4 producing a fusion gene (PDGRFA), among the causes for HES, can be treated with low dose imatinib (100-200 mg/week), thereby avoiding drug toxicity.
A 58-year-old retired male construction worker was referred to our institution with a diagnosis of HES presenting with nonspecific symptoms of jaw pain, nocturnal diaphoresis and general malaise. On first admission, he was found to have significant leukocytosis of 56.9×109/L with eosinophilia of 29.6×109/L. Past medical history was unremarkable and no recent changes to medication were reported. Family history for rheumatologic or malignant conditions was negative, as were travel history, farm stock exposure and allergies.
Neurological examinations showed normal colour vision, loss of smooth visual pursuit, positive saccadic movements and vertical skew deviation with difficulties describing the content of all eight visual fields. All other cranial nerves tested normal. Cerebellar examination revealed bilateral past-pointing, bilateral dysmetria, dysdiadochokinesia and positive left-sided heel-shin testing. Interestingly, all of his cerebellar symptoms improved following bilateral eye closure, indicating a component of visual ataxia. Moreover, he was noted to demonstrate right-sided sensory neglect, bilateral astereognosis, agraphesthaesia and simultagnosia which were suggestive of a rare presentation known as “Balínt Syndrome”.
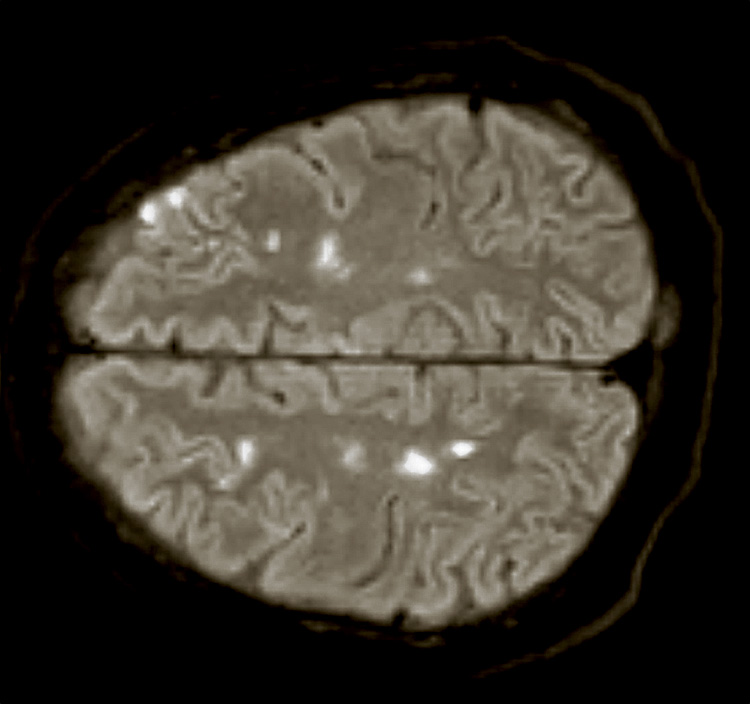
Subsequent imaging revealed bilateral supra- and inferior-tentorial white and grey matter hypodensities. Additional MRI imaging showed multiple areas of bilateral acute and subacute ischaemia, predominantly following a watershed distribution (Fig. 1). Both echocardiography and cardiac MRI showed evidence of global left ventricular dysfunction (LVEF 44%), however, no hemodynamically significant valve disease or thrombus were found.
Normal IgE, tryptase levels and lack of compelling history ruled out a hypersensitivity or parasitic aetiology. Autoimmune markers including ANA, ANCA, RF, and ACPAs were normal. Also, serum protein electrophoresis, flow cytometry, a peripheral blood smear and a lumbar puncture were not diagnostic.
Repeat blood work showed progressive rising leukocytosis and eosinophilia to a peak of 80.6×109/L and 45.6×109/L, respectively, eight days following admission. The patient was empirically started on high dose methylprednisolone (125mg PO daily). His visual field defects recovered, power in all his limbs returned and his simultagnosia resolved completely. His residual deficit comprised bilateral dysmetria, intention tremor and mild past pointing. Upon discharge, methylprednisolone was switched to prednisone (75mg PO daily).
Eventually, a bone marrow aspirate taken shortly after admission indicated a FIP1L1-PDGFRA translocation as the underlying aetiology. Following discharge, steroids and hydroxyurea were switched to imatinib due to hydroxyurea-induced cytopenia, upon which his eosinophil count returned to near-normal range within one week.
Discussion
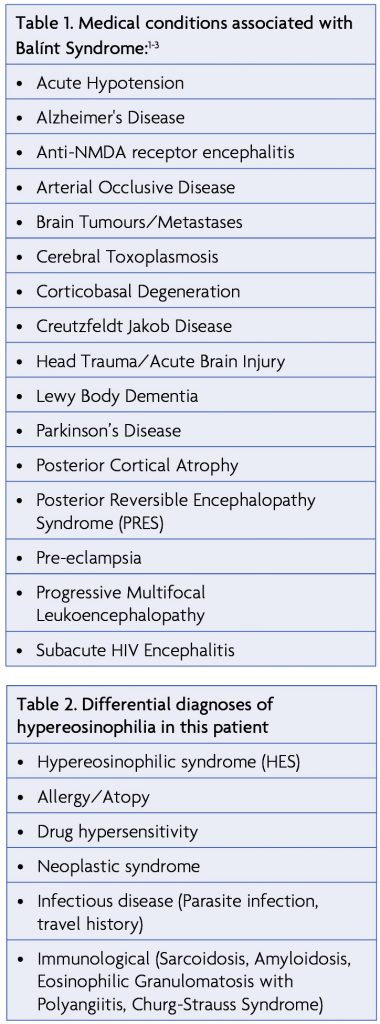
Considered a rare occurrence, Balínt Syndrome has been described in cases of ischaemia to both posterolateral occipital lobes (visual association area), the parieto-occipital junction and both posterior aspects of the parietal lobes.1 These areas of the brain are particularly vulnerable to watershed infarcts as they represent the locations of terminal branches of both the middle and posterior cerebral artery and can frequently become under-perfused in arterial occlusive disease or acute hypotension.2,3 Other occurrences of Balínt syndrome are listed in Table 1. It has been suggested that Balínt Syndrome results from lesions to specific functional brain areas responsible for reaching, saccades, grasp, attention and state estimation. Damage to the parieto-occipital junction, responsible for coordinating visual and hand movements, has therefore been postulated as a prime cause of optic ataxia while some forms of simultagnosia have been observed in inferior occipito-temporal lobe lesions.3
HES is a rare condition defined as persistent marked serum eosinophilia (>1.5×109/L for more than six months) alongside evidence of eosinophilia-induced organ damage. Other causes contributing to eosinophilia such as allergic reactions, parasites or malignant disorders should be excluded beforehand.4
For decades HES had been termed idiopathic. However, recent work could identify a novel kinase derived from a mutational deletion on chromosome 4 producing a fusion gene (FIP1L1-PDGFRA). As this mutation can be expressed in multiple cell lineages, increased serum levels of neutrophils and tryptase levels can frequently be observed. Patients displaying this chromosomal abnormality are classified as having chronic eosinophilic leukemia (CEL).4
Clinical manifestations of HES can be broad. However, major end organs affected by HES compromise the heart, skin, respiratory apparatus and central nervous system (CNS), specifically encephalopathy, peripheral neuropathy or focal CNS deficits from either thromboembolism or hemorrhage.4,5 The effects of eosinophils and their products on the coagulation cascade are not well understood, however, fibrinous material on the endocardial surface is an optimal location for thrombus propagation.4
In the present case, our patient presented with mild troponin elevation (1.7 ng/ml) in the absence of chest pain or shortness of breath. Cardiac involvement in HES occurs in three stages; an acute necrotic stage, followed by thrombotic, and finally fibrotic stages.6 During the acute necrotic stage, the endocardium and myocardium are infiltrated by eosinophils and lymphocytes resulting in necrosis, eosinophilic degranulation, and micro-abscess formation. Micro-emboli formation at the site of eosinophil degranulation-induced endothelial necrosis is known to give rise to early signs such as splinter hemorrhages.4,6 Given troponin elevation, evidence of reduced ventricular function without evidence of intraventricular thrombus or overt fibrosis, and MRI evidence of diffuse subendocardial late gadolinium enhancement, it is likely that our patient experienced the acute necrotic stage of cardiac involvement. Unseen by both echocardiography and cardiac MRI, cardiac micro-emboli becoming dislodged and swept into cerebral arteries are a likely explanation for the bilateral cortical watershed infarcts thereby giving rise to the clinical triad of Balínt Syndrome.
Regarding the specific rehabilitation of Balínt syndrome, treatment approaches are multifaceted as visual disturbances can take place both at lower and/or higher function levels. Moreover, spontaneous recovery in this condition is assumed to be low. However, individual case reports pointing out different rehabilitation protocols have come to show similarity among their treatment approaches. One of them is applying visuo-perceptual retraining and a functional adaptation program to restore functionality.7
Although still very incomplete, a scientific foundation about the rehabilitation of this condition is currently growing and there are approaches giving valuable information that can be built upon in the future.
Post-discharge, our patient continued to demonstrate slow rehabilitation progress in the strength in his arms with dysmetria on finger-to-nose testing and persistence of mild lower extremity discoordination with inability to perform tandem gait. EMG showed axonal predominant neuropathy thought to be secondary to his eosinophilia. He continues to see an ophthalmologist for mild visual impairment causing difficulty reading. Functionally, he continued to require the use of a walker as a gait aid for unsteadiness due to ataxia. He noted some residual confusion with mild difficulty retaining new memories, but his cognition remained largely unchanged.
Conclusion
To our knowledge, Balínt Syndrome has not been previously described in association with HES. This current case of Balínt Syndrome secondary to HES highlights the importance of watershed infarcts being a potential complication of acute hypereosinophilia-induced organ damage. In particular, the heart remains a location of micro-emboli formation which might not be detectable by cardiac imaging.
References
- Chechlacz M. Bilateral parietal dysfunctions and disconnections in simultanagnosia and Balint syndrome. Handb Clin Neurol. 2018;151:249-67. https://doi.org/10.1016/B978-0-444-63622-5.00012-7
- Kumar S, Abhayambika A, Sundaram AN, Sharpe JA. Posterior reversible encephalopathy syndrome presenting as Balint syndrome. J Neuroophthalmol. 2011;31(3):224-7. https://doi.org/10.1097/WNO.0b013e31821b5f92
- Andersen RA, Andersen KN, Hwang EJ, Hauschild M. Optic ataxia: from Balint’s syndrome to the parietal reach region. Neuron. 2014;81(5):967-83. https://doi.org/10.1016/j.neuron.2014.02.025
- Gleich GJ, Leiferman KM. The hypereosinophilic syndromes: current concepts and treatments. Br J Haematol. 2009;145(3):271-85. https://doi.org/10.1111/j.1365-2141.2009.07599.x
- Moore PM, Harley JB, Fauci AS. Neurologic dysfunction in the idiopathic hypereosinophilic syndrome. Ann Intern Med. 1985;102(1):109-14. https://doi.org/10.7326/0003-4819-102-1-109
- Mankad R, Bonnichsen C, Mankad S. Hypereosinophilic syndrome: cardiac diagnosis and management. Heart. 2016;102(2):100-6. https://doi.org/10.1136/heartjnl-2015-307959
- Heutink J, Indorf DL, Cordes C. The neuropsychological rehabilitation of visual agnosia and Balint’s syndrome. Neuropsychol Rehabil. 2018:1-20.




