Abstract
Posterior Reversible Encephalopathy Syndrome resulting from the hypertension-induced failure of cerebral autoregulation, is a well-described neuro-imaging finding resulting from vasogenic oedema. Pontine Hyperintensities resulting from this condition need recognition to prognosticate and avoid unnecessary investigations. We report a 48-year-old male with chronic diabetes mellitus, Hypertension, and chronic kidney disease, and history of liver transplantation who presented with established status epilepticus. He was on Tacrolimus for prophylaxis for graft rejection. His MRI brain showed diffuse pontine and predominantly left thalamic hyperintensity, which suggested the diagnosis of central PRES. His evaluation for CNS infections and autoimmune encephalitis was negative. On stopping Tacrolimus, the imputed drug, and control of hypertension, along with dialysis, and symptomatic management for seizures, a complete recovery was observed over one week. Repeat MRI also showed partial regression of the pontine hyperintensity. This report documents the importance of this less described neuroradiological finding that can change the management significantly and have a bearing on the prognosis.
Key learning points
- Diffuse non-restricted pontine Hyperintensity is a significant clue towards the rare central PRES.
- Tacrolimus, Hypertension, CKD are a few of the underlying factors to be looked for and corrected.
- Serum tacrolimus levels may not correlate with the clinical picture of neurotoxicity. Stopping it might be beneficial despite normal serum drug levels.
Introduction
Tacrolimus induced brain white matter abnormalities have long been described1. Posterior reversible encephalopathy syndrome (PRES), a clinico-radiological syndrome that can occur following tacrolimus administration, or other aetiologies, is also characterised in the literature. However, the typical description of PRES imaging is occipito-parietal white matter abnormality. There has been a flurry of literature recently dwelling on the atypical or central variant of PRES. This finding constitutes variable involvement of the brainstem and the basal ganglia. As this condition carries a good prognosis timely recognition is paramount. This case report emphasises recognising atypical brainstem involvement in patients who present with neurological complications while being on Tacrolimus.
Case presentation
A Forty-eight-year-old male presented to the casualty unit with status epilepticus. Seizures started the morning of admission, and he was brought to the hospital within about half an hour of onset. Seizures persisted despite the administration of phenytoin, Levetiracetam. He was a post-liver-transplantation patient (post alcoholic liver cirrhosis), with chronic renal failure, on Tacrolimus 0.5 mg twice a day, and mycophenolate mofetil 750 mg twice a day for the last four years. He also had diabetes mellitus, hypertension, and chronic kidney disease (CKD).
He was afebrile, comatose on admission, with no motor response on deep painful stimulation. There were no meningeal signs. His blood pressure at presentation was 190/100 mm Hg (Mean arterial pressure 130 mm Hg), the baseline being around 160/90 mm Hg. Pupils were symmetrical and reacting to light, and the doll’s eye response was preserved. Fundus evaluation revealed severe diabetic retinopathy, but no disc oedema.
Seizures subsequently were controlled after administration of lorazepam and loading dose of sodium valproate. Blood pressure was promptly controlled with intravenous labetalol infusion. Serum magnesium levels were 1.5 mEq/L, while the other electrolytes were normal on admission. Liver function tests were normal. Serum creatinine was 3.5 mEq/L, which was his baseline value. He had a haemoglobin of 7.7 gm/dL on admission. MRI brain and CSF evaluation were subsequently done.
On day 3 after admission, he developed reduced urine output, necessitating daily haemodialysis for about a week. Gradually his sensorium improved. On regaining consciousness, he did recollect headache in the morning of the day of seizures; however, he was amnestic about the events that followed. There were no focal neurological deficits on recovery.
Clinically, two diagnostic possibilities were considered initially: encephalitis, considering his immunosuppressed status; and hypertensive encephalopathy, considering the high blood pressure. He was evaluated for infectious and autoimmune encephalitis. CSF pressure was normal (20 cc of H2O), and CSF was acellular with mildly elevated proteins (49 mEq/L). Workup for CNS infections and autoimmune encephalitis was negative (Table 1).
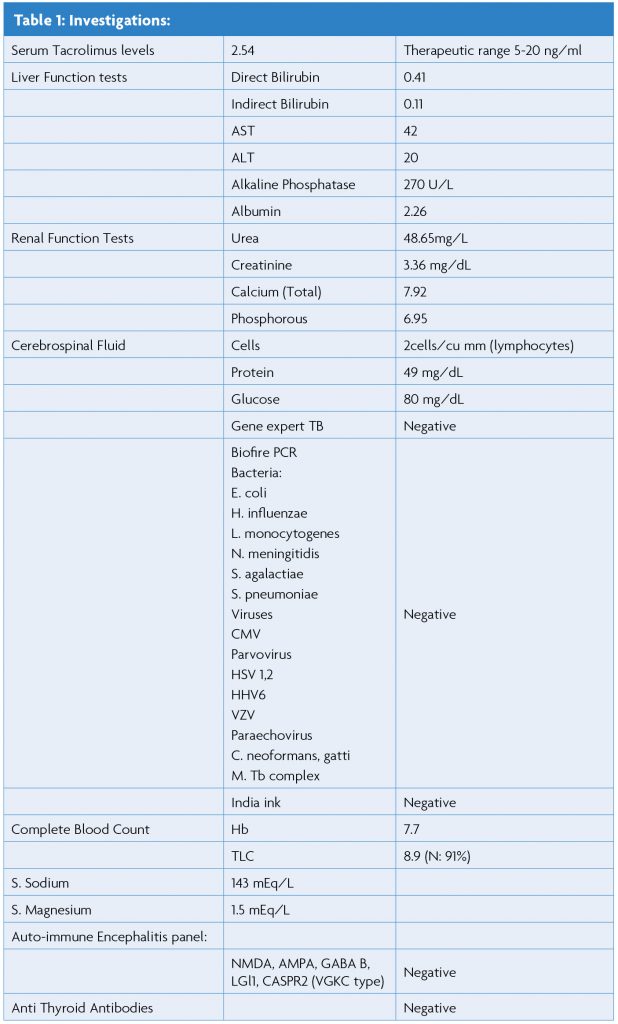
MRI brain helped us nail the diagnosis. It showed findings suggestive of non-restricted pontine and thalamic hyperintensity in the FLAIR and T2 weighted images. The clinical picture along with the imaging findings favoured the diagnosis of PRES.
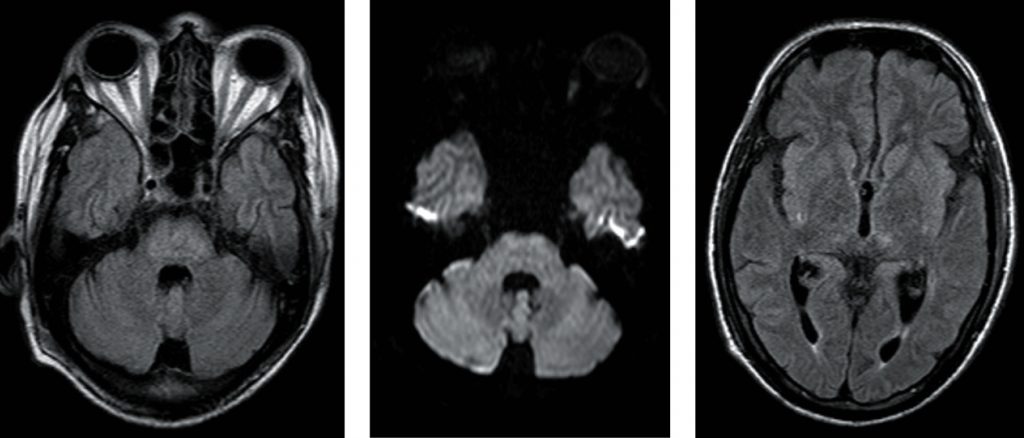
Diagnosis of PRES in this patient was confirmed by expression of central PRES pattern, as shown in Figures 1. a, b, and c with vasogenic oedema, reversibility on follow up (partial reversal after ten days in our case), as shown in Figures 2. a and b, along with the typical clinical presentation of PRES.
We considered Tacrolimus as an added imputing factor, in addition to hypertension and CKD. It was deemed an added attributing element, in addition to hypertension and CKD, and was, therefore, stopped. The patient discussed here had slightly low serum magnesium levels that were corrected after admission.
Discussion
Reversible Posterior Leukoencephalopathy Syndrome was initially described by Hinchey et al. back in 1996. They had noted patients with headache, altered sensorium, seizures, and visual deficits that completely reversed with treatment, along with the reversal of parieto-occipital white matter changes that correlated with the clinical picture.2 The clinical and radiological findings are attributed to subcortical vasogenic oedema resulting from a capillary leak from endothelial damage and disruption of the blood-brain barrier3. A myriad of causes and multiple comorbidities have been stated behind this disorder. These include hypertension, immunosuppressive drugs like calcineurin inhibitors, CKD, dialysis dependency, malignancy, and hypertension.2,3 Some studies describe an association of renal insufficiency and PRES; however, the mean arterial pressure in one study was 150 mm Hg.4 Hence, the real underlying cause could still be hypertension. Three patients in the case series by Hinchey et al., who developed PRES while being on Tacrolimus, had normal blood pressure. However, Hypertension and nephropathy are known with Tacrolimus as well as cyclosporine related CNS symptoms.2,4 Indeed, these side effects are more common than its neurotoxicity.5 Wong et al. noted hypertension in 2/10 patients on Tacrolimus who had developed PRES.6 According to a systematic review, hypertension was noted in 69% of calcineurin inhibitor-associated PRES, according to a systematic review.7 However, some authors state that more than 50% of the PRES induced by calcineurin inhibitors are not clinically hypertensive.8 There are; however, other calcineurin inhibitor mediated mechanisms that could result in this reversible vasogenic oedema. These include endothelial toxicity, resultant endothelin mediated vasoconstriction, micro thrombosis, and hemolytic uremic syndrome.2
Chen et al. describe a common immunogenic mechanism for PRES mediated by all causes like hypertension, immunomodulators, eclampsia, sepsis, and autoimmunity; this mechanism incites endothelial injury and involves cytokine release (IL 2, IL-1, TNF alpha, INF gamma), that result in T cell activation and upregulation of adhesion molecules like ICAM-1, VCAM-1, P and E selectins, and macrophage activation. Ultimately microcirculatory dysfunction, altered vascular tone, and impaired vascular permeability result.9 Both the cytotoxic (immunogenic) and vasogenic mechanisms could result in this condition.8 In the described patient, there is also a possibility of an immunogenic mechanism with an exacerbation induced by moderate hypertension (MAP 130 mm Hg).
Both these theories have been described in the pathogenesis of PRES, with the vasogenic theory supporting the MAP rise of beyond 150-160 mm Hg that contributes to PRES.8,3 There is another neuropeptide theory that explains the vasoconstriction resulting in cerebral oedema.3 Other mechanisms could also have contributed in our case. For example, the association of hypomagnesemia and PRES is not proven with Tacrolimus, however is known to contribute to cyclosporine-induced PRES; it could have been an undetermined inciting factor in the reported patient.10,11
Tacrolimus is a calcineurin inhibitor (FK506), an effective immunosuppressor for the prevention of graft rejection in solid organ and bone marrow transplantation. Most of the Tacrolimus induced PRES patients described in the literature had normal serum tacrolimus levels, and these levels failed to correlate with the degree of neurological symptoms.7 This could be explained by the variable half-life (3.5-40.5 hr) of Tacrolimus and active metabolites of Tacrolimus.1 Variability in Tacrolimus levels and lack of association of Tacrolimus levels with PRES has been described by other authors as well.12,13 One of these patients had renal insufficiency, possibly Tacrolimus induced.13 In a study, the incidence of Tacrolimus associated PRES was 1.6%.6 However, pontine PRES resulting from Tacrolimus has fewer reports. We could only find two case reports, one with reversible expressive aphasia, and the other one with diplopia and bilateral sixth nerve palsy.12,14
Posterior circulation predilection has been attributed to less sympathetic innervation that has a protective effect against unbridled cerebral vasodilation.15 Pons has also been purported as prone to vasogenic edema, particularly in CKD patients, as in our case, owing to albuminuria.16 Although brainstem findings have been noted in about 13% of PRES patients, this condition’s identification becomes easier because of co-existent hemispheric features. Although an equal expression (20-30%) of the three described primary imaging patterns in PRES with patchy/confluent or linear involvement (holo-hemispheric, superior sulcus pattern, dominant parietal-occipital pattern, and partial/ asymmetric pattern) is described9, an isolated pontine involvement is quite rare. McKinney et al. had initially described this isolated finding as central PRES, in a patient with cocaine toxicity who lacked the classical parietooccipital hyperintensity, but had brainstem, thalamic and deep white matter edema3. They subsequently reported about 4% of all PRES patients with similar findings9.
Although brainstem findings have been noted in about 13% of PRES patients, this condition’s identification becomes easier because of co-existent hemispheric features. Although all the three described imaging patterns in PRES with patchy/confluent or linear involvement (holo-hemispheric, superior sulcus pattern, dominant parietal-occipital pattern, and partial/asymmetric expression of these primary patterns have been described in almost equal proportions (20-30%),17 isolated pontine involvement is quite rare. McKinney et al. had initially described this isolated finding as central PRES and reported about 4% of all PRES patients with that finding.18
Conclusion
To conclude, Central PRES is a rare condition that otherwise presents with symptoms concordant with the commonly described PRES. People with organ transplantation receiving calcineurin inhibitors like Tacrolimus should be suspected with this diagnosis when they present with neurological symptoms. It is a completely reversible condition, when recognised and treated in time.
References
- Appignani BA, Bhadelia RA, Blacklow SC, Wang AK, Roland SF, Freeman RB. Neuroimaging findings in patients on immunosuppressive therapy: experience with tacrolimus toxicity. American Journal of Roentgenology 1996 Mar;166(3):683-8. https://doi.org/10.2214/ajr.166.3.8623651
- Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A Reversible Posterior Leukoencephalopathy Syndrome. New England Journal of Medicine. [Internet]. 1996;334(8):494-500. https://doi.org/10.1056/NEJM199602223340803
- Granata G, Greco A, Iannella G, Granata M, Manno A, Savastano E, et al. Posterior reversible encephalopathy syndrome-Insight into pathogenesis, clinical variants, and treatment approaches. Autoimmunity Reviews. 2015;14(9):830-6. https://doi.org/10.1016/j.autrev.2015.05.006
- Gao B, Liang H, Liu F, Lv C. Isolated Pons Involvement in Posterior Reversible Encephalopathy Syndrome in a Patient with Chronic Renal Insufficiency: Case Report and Literature Review. Clinical Neuroradiology. 2012 Dec https://doi.org/10.1016/j.ensci.2016.11.008
- Bechstein WO. Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transplant International. 2000;13(5):313-26. https://doi.org/10.1007/s001470050708
- Wong R, Beguelin GZ, Lima MD, Giralt SA, Hosing C, Ippoliti C, et al. Tacrolimus-associated posterior reversible encephalopathy syndrome after allogeneic haematopoietic stem cell transplantation. British Journal of Haematology [Internet]. 2003;122(1):128-34. https://doi.org/10.1046/j.1365-2141.2003.04447.x
- Song T, Rao Z, Tan Q, Qiu Y, Liu J, Huang Z, et al. Calcineurin Inhibitors Associated Posterior Reversible Encephalopathy Syndrome in Solid Organ Transplantation. Medicine (Baltimore) [Internet]. 2016 Apr 8 [cited 2020 Jun 9];95(14). https://doi.org/10.1097/MD.0000000000003173
- Gao B, Lyu C, Lerner A, McKinney AM. Controversy of posterior reversible encephalopathy syndrome: what have we learnt in the last 20 years? Journal of Neurology, Neurosurgery & Psychiatry. 2018;89(1):14-20. https://doi.org/10.1136/jnnp-2017-316225
- Chen Z, Shen G-Q, Lerner A, Gao B. Immune system activation in the pathogenesis of posterior reversible encephalopathy syndrome. Brain Research Bulletin 2017 May [cited 2020 Jun 17];131:93-9. https://doi.org/10.1016/j.brainresbull.2017.03.012
- Almoussa M, Goertzen A, Brauckmann S, Fauser B, Zimmermann C. Posterior Reversible Encephalopathy Syndrome due to Hypomagnesemia: A Case Report and Literature Review. Case Reports in Medicine. 2018;29;2018:1-6. https://doi.org/10.1155/2018/1980638
- Thompson CB, Sullivan KM, June CH, Thomas ED. Association between cyclosporin neurotoxicity and hypomagnesaemia. The Lancet. 1984 Nov:1116-20. https://doi.org/10.1016/S0140-6736(84)91556-3
- Oliverio PJ, Restrepo L, Mitchell SA, Tornatore CS, Frankel SR. Reversible Tacrolimus-induced Neurotoxicity Isolated to the Brain Stem. American Journal of Neuroradiology. 2000 Aug 1;21(7):1251-4. https://pubmed.ncbi.nlm.nih.gov/10954277/
- Small SL, Bramblett GT, Eidelman BH, Fukui MB. Immunosuppression-induced leukoencephalopathy from Tacrolimus (FK506). Annals of Neurology. 1996;40(4):575-80. https://doi.org/10.1002/ana.410400406
- Jorge R, Gayowski T, Fung J, Todo S, Alessiani M, Starzl TE. Expressive Dysphasia Possibly Related to Fk506 in Two Liver Transplant Recipients. Transplantation. 1990;50(6):1043-81. https://doi.org/10.1097/00007890-199012000-00028
- Beausang-Linder M, Bill A. Cerebral circulation in acute arterial hypertension-protective Hypertension effects of sympathetic nervous activity. Acta Physiologica Scandinavica [Internet]. 1981;111(2):193-9 https://doi.org/10.1111/j.1748-1716.1981.tb06724.x
- Gowan JM, Liu A. Isolated pan-pontine posterior reversible encephalopathy syndrome in a patient with uncontrolled hypertension. Clinical Case Reports 2019;7(1):32-6. https://doi.org/10.1002/ccr3.1888
- Bartynski WS, Boardman JF. Distinct Imaging Patterns and Lesion Distribution in Posterior Reversible Encephalopathy Syndrome. American Journal of Neuroradiology [Internet]. 2007 Aug 1;28(7):1320-7. https://doi.org/10.3174/ajnr.A0549
- McKinney AM, Jagadeesan BD, Truwit CL. Central-Variant Posterior Reversible Encephalopathy Syndrome: Brainstem or Basal Ganglia Involvement Lacking Cortical or Subcortical Cerebral Edema. American Journal of Roentgenology. 2013 Aug 23;201(3):631-8. https://doi.org/10.2214/ajr.166.3.8623651

