On December 7 – 9, 2015, the annual Human Induced Pluripotent Stem Cell (iPSC) was held in the Department of Medical Sciences at the University of Oxford, led by Dr Sally Cowley, Head of the James Martin Stem Cell Facility. The course was aimed at scientists and doctors looking to adopt iPSCs as a tool for their own research endeavours, and was an in-depth practical and theoretical course seeking to provide the skills necessary to derive and expand these cell lines as well as to differentiate them into certain lineages.
Since Yamanaka discovered that terminally differentiated adult somatic cells can be reprogrammed into stem cells using just four additional factors, iPSCs have become an increasingly popular and are now an essential tool for medical research. In particular, the main advantage iPSCs provide over other cell lines is that it enables a specific patient’s own cells to be studied in vitro, allowing modelling of patient-specific disease – an invaluable tool. The first day of the course provided the historical and theoretical background behind iPSCs before the first practical workshop in the cell culture room, in which delegates observed growing iPSCs in culture and learned how to select single colonies for deriving clonal lines.
The meeting offered many sessions to suit interests of scientists from diverse backgrounds. For example, there was a dedicated session on differentiating iPSCs into pancreatic beta-cells, particularly useful for studying mechanisms of disease of patients with genetically-driven diabetes. However, for neuroscientists perhaps the most exciting sessions were those on the use of iPSCs for studying neurological disease. The first such session was delivered by Dr Zameel Cader, a neurologist in Oxford specialising in pain disorders. He gave cogent arguments on why iPSCs would be especially useful for studying neurological disorders – the fact that the nervous system has a more limited capacity for self-repair compared with other tissues means that stem cells holds promise for regenerative medicine, and that clinical trials in neurology are more expensive and prone to failure compared with other disease and therefore accurate models of disease are desperately needed (which iPSCs now potentially provide). The talk outlined the steps in differentiating stem cells into sensory neurons, with confirmation of neuronal identity using antibody staining, electrophysiological measurement for mature action potentials and RT-PCR for neuronal markers. Such work promises to help in modelling chronic pain disorders and drug screening.
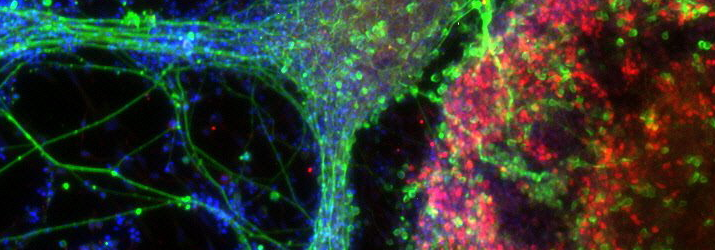
Continuing with the theme of deriving models of neurological disease, Dr Colin Akerman delivered an insightful session on his research on using iPSCs from patients to derive human cortical neurons in vitro. The most exciting aspect of this work is that both excitatory and inhibitory neurons can be produced using recently established protocol, and moreover that synapses between these neurons form in vitro and are fully functional, validated with detailed electrophysiology. This is important because much work has looked at neuronal degeneration in disease but studying synaptic abnormalities has been more difficult to do. Further novel insights may come from optical techniques for monitoring synaptic transmission, for example with calcium imaging, and for manipulating synaptic transmission using light-activated proteins in these cortical neurons. Closely linked to this session was another delivered by Dr Sarah Newey from the Department of Pharmacology, University of Oxford. Using similar tools as Dr Akerman, her interests are in iPSCs for providing culture models of neurodegenerative disease. She described protocols for taking iPSCs from patients with Alzheimer’s disease and differentiating them into cortical progenitors and neurons, and the cell lines made in this way are remarkably reproducible between different laboratories. This approach promises to be useful for drug screening of Alzheimer’s disease. A practical session was conducted in which delegates experienced first-hand how to perform antibody staining for cortical progenitors and neurons in order to confirm successful differentiation from iPSCs, and photographs were taken of these neurons under the microscope for delegates to take away with them.
Induced pluripotent stem cells are increasingly being used for a diverse range of research purposes and allow unprecedented study of disease at the level of individual patients. The course was very well planned and conducted, with all attendees having learnt much about the applications of this tool as well as learning the practical skills necessary for establishing and differentiating these cells in their own laboratories.
ACNR 2015;16(2): 30. Published online 14/6/16
To Cite: Noorani I, ACNR 2016; 16 (1)