
ECCO ESMO is the premier conference held in Europe which is attended by clinicians involved in the management of cancer from all over the world.
Building on the previous successes,this year it was organised by ECCO in partnership with ESTRO, ESMO, ESSO, EACR,EONS and SIOPE,a true multidisciplinary gathering.As expected,large numbers of professionals gathered in Amsterdam, one of the most beautiful and charming cities in Europe, from every discipline of oncology to share their ideas, present ground.breaking results from ongoing research and discuss the best way forward to implement practice-changing evidence on a single platform to conquer the dreadful disease: brain tumours. More than 18,000 delegates attended and over 3300 abstracts were presented. The theme of 2013 conference was genomics and multidisciplinary approach. High quality debates, multiple educational lectures and symposia on topical subjects turned out to be some of the most popular events. It was understandable that the data presented were heavily loaded in favour of tumours like breast, lung, colorectal and urological cancers.However,other primary tumour sites like nervous system, malignant melanoma and gynaecological cancers were also well represented.
Malignant glioma, one of the common central nervous system (CNS) primary tumours, has a very aggressive behaviour and limited success leading to poor outcomes with currently available treatment modalities for majority of unfortunate sufferers. Most of the glioblastoma patients eventually experience relapse. Management of these individuals is complicated by the fact that re.treatment with high dose radiotherapy can risk radiation induced critical damage to the normal brain tissue. Options for further chemotherapy may also be limited due to the development of drug resistance. Therefore, use of currently available chemotherapy agents have been of limited value resulting in poor quality of life and a fatal outcome sooner rather than later.
It is now apparent that malignant glioma is a heterogeneous entity.Therefore,there has been considerable interest to find a new approach particularly molecular profiling, detection of potential biological drivers and the use of biological agents in its management. Survival prediction of the outcome with chemotherapy depends on molecular characteristics such as deletion of chromosomes 1p and 19q, mutations of IDH1/2 and methylation of repair enzyme MGMT. The significance of the mutation of IDH genes in gliomas is growing and the future of their place in the development and management of anaplastic glioma is becoming clearer. The IDH1 gene encodes cytoplasmic, while IDH2 encodes mitochondrial enzyme isocitrate dehydrogenase.The IDH1 mutation is more common (40%) compared to IDH2 in gliomas. Both these mutations are associated with 1p and 19q co-deletion. The presence of these mutations carries a better prognosis in all grades of glioma but its predictive value remains unknown.Accumulated D-2-HG can be detected by MR-spectroscopy and could be utilised as a diagnostic marker. Inhibitors of mutated IDH1 and IDH2 have shown reversal of various epigenetic hyper-methylation changes. Therefore, they could be useful therapeutic targets.
In addition, the CD95 signalling pathway has also been recently identified to be an important signalling pathway in tumour progression. In a multicentre, phase II trial, 84 recurrent glioblastoma patients were randomised to receive re-irradiation alone or re-irradiation plus weekly intravenous dose (400 mg) of APG101,a CD95/CD95L inhibitor fusion protein similar to an antibody known as APG101. The data presented by Prof Wolfgang Wick showed that 21% of the patients in the combination arm compared to only 4% in the re.irradiation alone arm were alive at six months. The 2 years survival was 22% for the combination compared to only 7% for control arm. It was encouraging to see that the risk of death was reduced by 40% in the experimental treatment group but it did not reach statistical significance.This is the first controlled trial of re-irradiation in glioblastoma.Although the size of the APG101 protein molecule is potentially too large to cross the protective blood-brain barrier, radiotherapy is considered to open up this barrier to target the tumour.Tumours expressing the CD95L protein carry poor prognosis and responded better to the APG101 combination treatment in this study. However, further research is needed to understand the exact mode of action and development of drug resistance, along with its use in CD95/CD95L over-expressing tumours as well as in combination with other agents like temzolomide. Abstract 3304
The MGMT gene is an important promoter of an important resistance factor against alkylating agents.With methylation there is an inactivation whilst with unmethylation there is an activation of this gene. Temozolomide has improved the survival in patients with newly diagnosed glioblastoma modestly and very marginally if the tumour MGMT was not methylated. Bevacizumab in combination with irinotecan (BEV-IRI) is approved and frequently used in USA for the management of relapsed glioblastoma. The clinical activity of this doublet led the clinicians to wonder whether similar benefits could be achieved by inhibiting tumour angiogenesis with bevacizumab in newly diagnosed glioblastoma, a deadly disease with limited treatment options. The results of GLARIUS, a phase II (n=182), randomised (2:1) trial comparing bevacizumab,irinotecan and radiotherapy with standard radiotherapy and temozolomide in MGMT-non-methylated newly diagnosed glioblastoma was reported to show remarkable improvement in the median progression.free survival (PFS) of 9.7 months with BEV-IRI compared with 6 months with the standard treatment (HR=0.3, p=<.0001).Similarly,a significant advantage was observed in overall survival in the BEV-IRI arm (16.6 vs. 14.8 months) compared to the standard arm. These preliminary results are promising and provide additional evidence to support the activity of irinotecan in this dreadful disease. Abstract 3300
Continuation of Bevacizumab (bev) beyond progression is another area of interest among the investigators following success observed in colorectal and ovarian cancers. Avaglio is the first randomised, double blind, placebo controlled, phase III study to evaluate the efficacy and safety of the addition of bev to standard temozolomide plus radiotherapy for newly diagnosed glioblastoma. The study met the co-primary end point of median PFS of 10.6 vs 6.2 months (HR=0.64; p <0.0001) in favour of the bev group. Clinical deterioration-free survival was also increased from 3.9 months to 6.4 months and time to deterioration, irrespective of the type of progression, increased from 5.6 months to 8.5 months. The Karnofsky-performance status (KPS) above 70 was maintained for a median duration of 6 months in the placebo arm compared to 9 months in the bev arm. Off steroids were 45% vs. 61% patients and adverse events observed in 51% vs. 66.8% in placebo and bev arm, respectively. Arterial thrombo-embolism increased in the bev arm (1.3% with placebo and 5% in the bev arm),but there was no difference in venous thrombo-embolism (8% with placebo and 7.6% with bev). Subsequent chemotherapy was delivered in 64% of patients in placebo and in 57% of patients in the bev arm.There was no difference in the pattern of progression. Abstract 3301A
The current standard of care for glioblastoma consists of concurrent radiotherapy-chemotherapy with temozolomide followed by 6 more cycles of maintenance temozolomide. Unfortunately, the median PFS and overall-survival (OS) remain extremely poor.
Significant improvement remains the unmet need in this extremely angiogenic malignancy. For the management of glioblastoma, integrin inhibition is probably one of the most important goals because it targets both pathological tumour vasculature as well as the direct tumour cells. Over 3500 newly diagnosed patients with glioblastoma were screened for MGMT methylation and in the Centric trial,patients with positive methylation (n=540) were randomised to be treated with standard radiotherapy and temozolomide with (n=272) or without (273) cilengitide, which is a selective .v.3 and .v.5 integrin inhibitor and interferes with cell attachment and migration. Cilengitide 2000mg intravenous twice weekly was administered and continued for up to 18 months or progression.It is important to note that there was no placebo therapy in the control arm which would have been unethical for patients to have been unnecessarily visiting the clinics for placebo infusion. The median survival was 26.3 months in cilengitide and 28 month in the placebo arms. Although median PFS was longer at 10.6 months in cilengitide arm compared to 7.9 months in the placebo,the difference was statistically not significant. Centric trial updates did not identify any subgroup which could have benefited from cilengitide either. Similarly, no major safety concerns were identified.Despite a negative outcome in this trial, integrin remains an attractive target. Abstract 3302
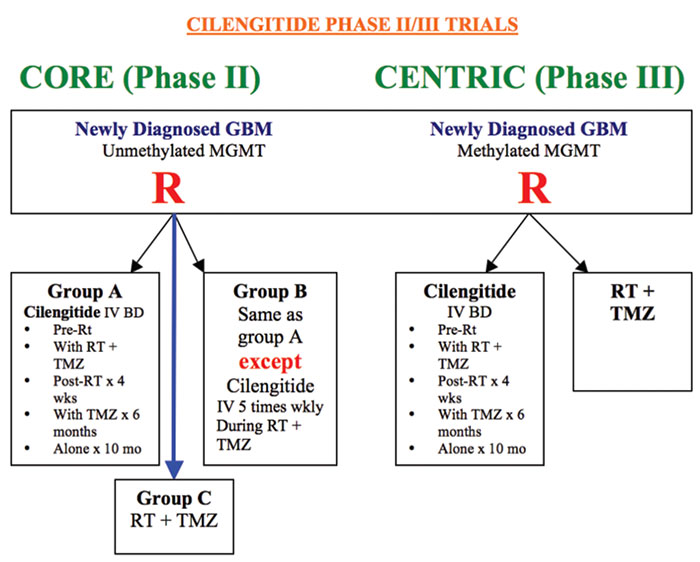
Whilst in the Centric trial,cilengitide was used in methylated GBM; CORE an ongoing phase II trial, used cilengitide in unmethylated tumours with the addition of two different dosage schedules (an additional intensive dose) of cilengitide infusion. In this multicentre, randomised, open-label study, compared to the control arm, there was no significant benefit between the three groups of patients.Abstract LBA 40
These results illustrate the challenging behaviour of primary brain cancer. The challenge is monumental but progress in scientific research and new discovery is relentless leading to rapid expansion of our knowledge. We have already achieved new mile stones for many other tumours and hope to celebrate the discovery of effective therapy for CNS tumours soon. The ESMO2013 clearly was a mega success. The organisers and speakers deserve admiration to be able to attract such a large audience and present many new ground breaking research data on a wide variety of tumours.The feather in their cape was the inclusion of many sessions focused specifically for oncology nurses and other support professionals.
The presentations are available in the form of webcast on ESMO site though there is a small charge but the readers would find them extremely valuable, particularly those who could not attend this wonderful meeting.
Abbreviations: European Cancer Organisation (ECCO), European Society of Medical Oncology (ESMO), European Society for Radiotherapy and Oncology (ESTRO), European Society of Surgical Oncology (ESSO), European Association of Cancer Research (EACR), European Oncology Nursing Society (EONS) and the European Society of Paediatric Oncology (SIOPE)

