Abstract
Myasthenia Gravis (MG) is an acquired, autoimmune disorder of the neuromuscular junction, resulting in fluctuating skeletal muscle weakness and fatigue. MG prevalence increases with advancing age and is estimated to be 12 per 100 000 in European females below age 50 years [1]. The interplay of MG and pregnancy poses unique challenges for pregnant women and healthcare providers. In the UK, managing MG during pregnancy involves a multidisciplinary approach, bringing together neurologists, obstetricians, anaesthetists, and neonatologists. This collaboration is critical given that about 30% to 40% of women with MG may experience a worsening of their symptoms during pregnancy [2]. This review seeks to highlight the current consensus-based practice in the UK regarding MG management in pregnancy.
Introduction
Neither MG, nor the commonly used drugs in MG are expected to influence fertility. Females with MG tend to have fewer children than healthy women, but this can be explained by reasons other than reduced fertility [3].
To minimise the effects of MG on pregnancy and the newborn, discussion about planned pregnancy is essential and needs to occur early. There should be close collaboration with obstetricians in the preconception stage as well as throughout pregnancy. The aim is for stable disease before conception whilst avoiding treatments associated with teratogenic effects.
Once pregnant, it is important to avoid factors that can cause MG exacerbation, such as giving certain drugs or withdrawing immunosuppression. Infections should be treated promptly, and thyroid status should be checked, if not done before. The clinical course can be variable and so patients need easy access to their myasthenia specialist team.
MG treatment during pregnancy
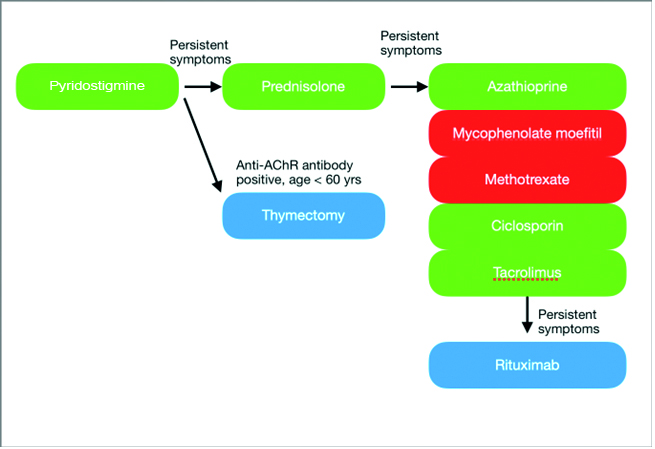
Figure 1. The recommended treatment pathway for MG [4,5,8]. The green therapies are considered safe in pregnancy, the red ones should be avoided, and the blue ones are safe depending on timing.
The recommended treatment pathway for MG is applicable for all patients with MG but during pregnancy, treatments with known teratogenic effects are avoided (see figure and table) [4,5].
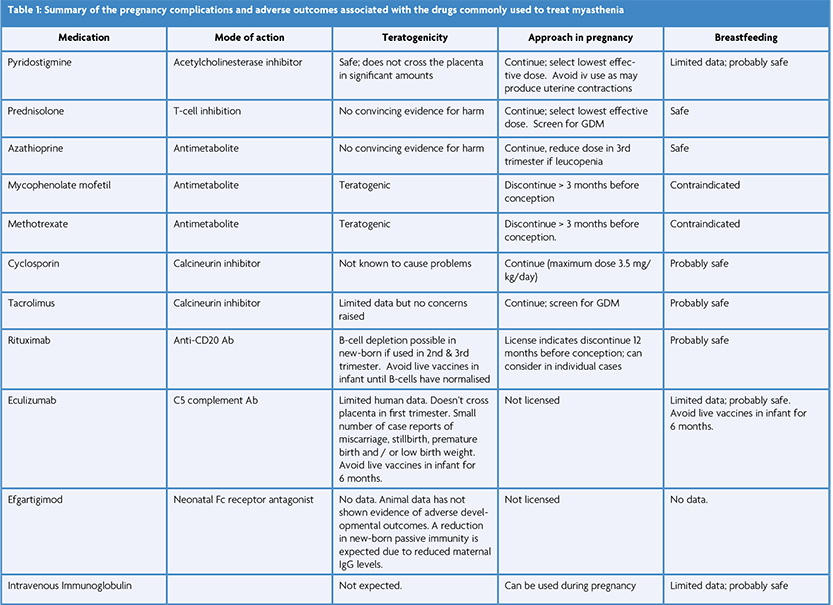
Pyridostigmine should be the initial treatment in most patients with MG with the dose adjusted as needed based on symptoms. Corticosteroids and/or non-steroidal immunosuppressive therapy should be introduced when symptoms are not adequately controlled by pyridostigmine alone.
Most females of reproductive age with MG and acetylcholine receptor (AChR) antibodies have an enlarged and hyperplastic thymus and thymectomy early in the disease improves outcome [6]. If possible, surgery should be performed well before pregnancy or otherwise postponed until after pregnancy. Thymectomy may reduce the risk of neonatal myasthenia [7].
Prednisolone and Azathioprine are considered safe in pregnancy [4]. Sometimes additional medications are prescribed to counteract corticosteroid side effects. Vitamin D supplements are recommended for all women during pregnancy and omeprazole is also commonly used. However, bisphosphonates are not usually recommended although the risks are largely unknown. Since bisphosphonates are stored in bone for up to 10 years, in theory a pregnancy occurring several years after bisphosphonate use could still be exposed.
Mycophenolate mofetil, methotrexate and cyclophosphamide are teratogenic immunosuppressive medications and so are generally avoided in all women of reproductive age, at least if there is a chance of pregnancy. The calcineurin inhibitors (e.g., ciclosporin) are considered safe in pregnancy but are generally used second line to azathioprine due to potential toxicity.
Recent advancements in clinical neuroscience have provided deeper insights into the pathophysiology of MG, paving the way for more targeted therapies. Notably, the development of monoclonal antibodies like rituximab and eculizumab have resulted in significant improvement in MG patients. Their safety in pregnancy remains under investigation and timing of dosing is important to prevent significant immunosuppression in the new-born [8].
Plasma exchange (PLEX) and intravenous immunoglobulin (IVIg) are the mainstay of management in myasthenia crisis and are safe to use in pregnancy.
Effect on mother’s MG
The relative risk for onset of MG during pregnancy is not increased but there is a 5-fold increased risk of onset of MG during the first 6 months postpartum [4]. 30-40% of women with MG may experience worsening of their symptoms during pregnancy but the clinical course is variable and difficult to predict, even from pregnancy to pregnancy in the same woman. Exacerbations are generally mild – moderate and again, occur more commonly during the first 6 months postpartum [4]. Myasthenic crisis is rare.
Anatomical changes during pregnancy may worsen pre-existing myasthenia symptoms. Pregnancy increases the risk of gastric reflux and those with associated bulbar involvement are at particular risk of subsequent pulmonary aspiration as they are less able to clear secretions and protect their airway. Respiratory muscle weakness in patients with MG can exacerbate the normal reduction in functional residual capacity and volume that occurs in pregnancy which could precipitate respiratory failure.
Complications and adverse outcomes in pregnancy
Most women with MG generally experience uncomplicated pregnancies. However, research suggests that compared to their healthy counterparts, women with MG may have a slightly higher risk of pregnancy complications during delivery. The rates of these complications vary across different studies due to factors such as population, sample size, and study design. Potential complications of MG pregnancies include pre-term birth (12%) and low birth weight (9%), especially in cases where symptoms worsen during pregnancy [4]. To reduce the risk of birth defects, it is recommended that all pregnant women, including those with MG, supplement with folic acid.
Several studies have examined the adverse effects of corticosteroid exposure on pregnancy and birth outcomes. However, these studies are largely observational and fail to adequately explore confounding factors related to the disease or its severity [9]. Corticosteroid use is associated with an increased risk of gestational diabetes mellitus (GDM), elevated blood pressure, infections, and pre-term deliveries. Therefore, screening for GDM through a glucose tolerance test is recommended at 28 weeks of pregnancy or earlier if there are additional risk factors present, such as hypothyroidism or a high body mass index (BMI) [3].
For women with MG, vaginal delivery is generally recommended, as the smooth muscle fibres of the uterus remain unaffected by the condition. While a higher rate of Caesarean section is reported, possibly due to the contribution of striated (skeletal) muscle in the second stage of labour, it should only be performed for obstetric indications and not solely as a precaution to prevent exhaustion. Nitrous oxide (Entonox) can be used as usual, and epidural analgesia is preferred over general anaesthesia. Although most anaesthetic drugs are safe for women with MG, they are highly sensitive to muscle relaxants. Pethidine and other opioids should be avoided, as they may worsen respiratory depression in both the mother and foetus. Additionally, mothers who receive more than 7.5 mg of long-term daily prednisolone should receive parenteral steroids to cover the stress of delivery [4].
In the event of complications, certain obstetric drugs should be avoided. Magnesium sulphate is not recommended for the management of eclampsia in women with MG due to its neuromuscular blocking effects.
Neonatal myasthenia
It is recommended to give birth at a hospital with experience in neonatal intensive care, as approximately 10% of babies born to mothers with MG may experience transient muscle weakness, typically within the first 24 hours after birth. This muscle weakness is reported most often to occur in babies of MG mothers with AChR antibodies but has also been described with muscle specific kinase (MuSK) antibodies, as well as in those without detectable muscle antibodies. It is caused by the transfer of pathogenic autoantibodies through the placenta. As the mother’s IgG antibodies break down in the baby, the muscle weakness improves, but it may take several weeks for normal function to be restored. There is no direct correlation between the maternal antibody titre or the severity of the mother’s MG and the risk of neonatal myasthenia [7]. However, having a previous child with neonatal myasthenia increases the risk of the condition in subsequent pregnancies [10].
Arthrogryposis, a rare and often fatal condition characterised by skeletal abnormalities and joint contractures, can occur in children of mothers with MG. This condition arises when the mother’s IgG antibodies bind to the foetal-type AChR, leading to restricted foetal movements in utero. In even rarer cases, a persistent myopathy termed “foetal acetylcholine receptor inactivation disorder” occurs, which is attributed to a permanent change in the post-synaptic membrane caused by maternal AChR antibodies during a critical period of foetal development. Maternal treatment with IVIG or PLEX can inhibit the development of arthrogryposis. Therefore, close monitoring of foetal movements is important for all women with MG to detect any abnormalities [10].
Breast feeding
Breast feeding is not known to influence mothers’ MG and should be encouraged as there are many advantages, including reducing the risk for autoimmune disease later in life [11]. Maternal IgG levels in breast milk comprise only 2% of that in serum and therefore would not be expected to cause harm.
Maternal transfer of pyridostigmine, prednisolone, azathioprine or their metabolites into breast milk is minimal and breastfeeding is probably safe also for treatment with monoclonal antibodies (e.g., Rituximab) and the calcineurin inhibitors. Known teratogenic MG medications should not be given to mothers with MG who are breast feeding.
Conclusion
MG does not represent a reason for not having children, and the patients should be supported in their wish of becoming pregnant.
Myasthenia gravis in pregnancy demands a delicate balance between managing maternal symptoms and ensuring foetal safety. Understanding these complexities, recognising potential complications, and staying abreast of emerging therapies are crucial to optimising outcomes for both mother and child. Further research is warranted to better guide the management of this challenging intersection of neurology and obstetrics.
Read the first article in this series: Myasthenia Gravis – a new era of treatment options | ACNR
References
- Andersen JB, Heldal AT, Engeland A, Gilhus NE. Myasthenia gravis epidemiology in a national cohort; combining multiple disease registries. Acta Neurologica Scandinavica. 2014;129(10:26-31. https://doi.org/10.1111/ane.12233
- Gilhus NE. Myasthenia Gravis Can Have Consequences for Pregnancy and the Developing Child. Front Neurol. 2020;11:554. https://doi.org/10.3389/fneur.2020.00554
- Ohlraun S, Hoffmann S, Klehmet J, Kohler S, Grittner U, Schneider A, et al. Impact of myasthenia gravis on family planning: How do women with myasthenia gravis decide and why? Muscle Nerve. 2015;52(3):371-379. https://doi.org/10.1002/mus.24556
- Norwood F, Dhanjal M, Hill M, James N, Jungbluth H, Kyle P, et al. Myasthenia in pregnancy: best practice guidelines from a UK multispecialty working group. J Neurol Neurosurg Psychiatry. 2014;85(5):538-543. https://doi.org/10.1136/jnnp-2013-305572.
- Sussman J, Farrugia ME, Maddison P, et al. Myasthenia gravis: Association of British Neurologists’ management guidelines. Pract Neurol. 2015;15(3):199-206. https://doi.org/10.1136/practneurol-2015-001126
- Wolfe GI, Kaminski HJ, Aban IB, Minisman G, Kuo HC, Marx A, et al. Long-term effect of thymectomy plus prednisone versus prednisone alone in patients with non-thymomatous myasthenia gravis: 2-year extension of the MGTX randomised trial. Lancet Neurol. 2019;18(3):259-268. https://doi.org/10.1016/S1474-4422(18)30392-2
- Gveric-Ahmetasevic S, Colic A, Elvedji-Gasparovic V, Gveric T, Vukelic V. Can neonatal myasthenia gravis be predicted? J Perinat Med. 2008;36(6):503-506. https://doi.org/10.1515/JPM.2008.070
- Dobson R, Rog D, Ovadia C, et al. Anti-CD20 therapies in pregnancy and breast feeding: a review and ABN guidelines. Practical Neurology 2023;23:6-14. https://doi.org/10.1136/pn-2022-003426
- Bandoli G, Palmsten K, Forbess Smith CJ, Chambers CD. A review of systemic corticosteroid use in pregnancy and the risk of select pregnancy and birth outcomes. Rheum Dis Clin North Am. 2017;43(3):489-502. https://doi.org/10.1016/j.rdc.2017.04.013
- Borba VV, Sharif K, Shoenfeld Y. Breastfeeding and autoimmunity: Programing health from the beginning. Am J Reprod Immunol. 2018;79(6):e12778. https://doi.org/10.1111/aji.12778
- Borba VV, Sharif K, Shoenfeld Y. Breastfeeding and autoimmunity: Programing health from the beginning. Am J Reprod Immunol. 2018;79(6):e12778. https://doi: 10.1111/aji.12778
