Teriflunomide
Introduction
In the first of this 3-part series of articles, we looked at the first oral therapeutic for relapsing-remiting multiple sclerosis (RRMS) to gain approval – the sphingosine-1-phosphate receptor modulator fingolimod. Teriflunomide became the second oral therapy approved by the EMA in 2013. In January 2014 NICE recommended Teriflunomide for treating adults with active RRMS only if they do not have highly active or rapidly evolving severe (RES) RRMS.1 Teriflunomide is a once a day tablet, derived from leflunomide which has been used extensively in rheumatology. Teriflunomide has undergone an extensive trial development with three pivotal phase III studies examining both 7mg and 14mg dose, in Europe the licenced dose is 14mg. Part 3 of the series looks at Tecfidera.
Mechanism of Action
Teriflunomide, a de novo pyrimidine synthesis inhibitor, is the primary metabolite of leflunomide which has been used worldwide as an oral disease modifying drug in the management of rheumatoid arthitis since 1998.2 Although the mechanism of action is not fully understood, teriflunomide is believed to have both anti-inflammatory and anti-proliferative actions mediated via reversible inhibition of the mitochondiral enzyme dihydroorotate dehygrodenase (DHO-DH).3 This enzyme is needed for the de-novo synthesis of pyrimidines in actively dividing cells, particularly proliferating lymphocytes.
Rapidly absorbed with maximum concentrations achieved after 12 hours, teriflunomide is metabolically stable with a long half-life of 10-12 days.4-5 It undergoes metabolism primarily via hydrolysis with a small component via oxidation. Clinical data suggests that the majority of elimination is of unchanged product via faeces, followed by elimination of the metabolite 4-trifluoromethylaniline (TFMA) oxalinic acid via urine.6 As teriflunomide may remain detectable in the serum for many months after stopping treatment, elimination can be accelerated using a course of activated charcoal or cholestyramine. This may be used for women wishing to become pregnant and clearance of teriflunomide can be confirmed by measuring serum levels. 99% of the compound is protein bound (indicating high potential of drug interaction) however teriflunomide is not metabolised by cytochrome P450 (CYP).4
Clinical Efficacy
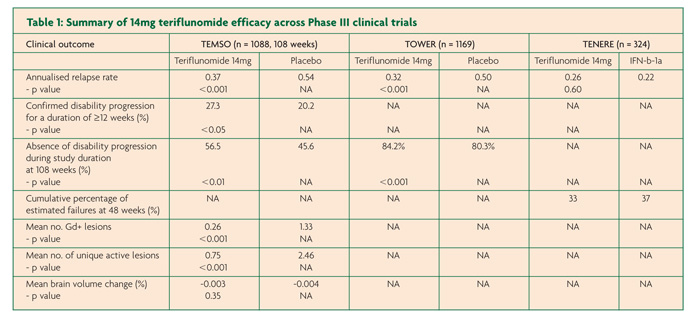
There have been three pivotal Phase III, international, multi-centre trials in the development programme of teriflunomide for RMMS; two placebo-controlled studies TEMSO and TOWER and the active-controlled TENERE that compared teriflunomide to injectable IFN-b (Rebif™).7-9 TEMSO, TOWER and TENERE each contained two teriflunomide arms at a daily dose of 7mg or 14mg. Only the results of the licensed dose – 14mg – will be presented here.
TEMSO contained 1088 patients over a study duration of 108 weeks.7 Annualised relapse rate (ARR) was the primary endpoint of the study with the results showing a relative risk reduction in relapses of 31.5% for the 14mg group compared to placebo (0.37 versus 0.54, p<0.001). The principle secondary end point of confirmed disease progression for at least 12 weeks was also significantly reduced for the teriflunomide arm compared to placebo (20.2% versus 27.3%, p≤0.05). MRI findings were encouraging with a 67.4% smaller change from baseline in total lesion volumes compared to placebo (+0.72 versus +2.21, p<0.001), fewer Gd-enhancing lesions on T1-weighted scans (0.26 versus 1.33, p<0.001) and fewer unique active lesions (0.75 versus 2.46, p<0.001). In contrast however, brain atrophy data found no difference between placebo and either teriflunomide arm.
TOWER contained 1169 participants from 26 countries but in this case treatment duration was variable with a range between 48-152 weeks.8 Again the primary end point of ARR was used and a relative risk reduction of 36.3% was found for the teriflunomide arm when compared with placebo; (0.32 versus 0.50, p=0.0001). The main secondary end-point was the time to sustained disability progression (defined as an increase in EDSS score by 1 point for at least 12 weeks) and only just achieved significance for the 14mg arm with a risk reduction of 31.5% compared to placebo (Hazard ratio versus placebo 0.68, P<0.05).
TENERE involved a smaller participant group of 324 patients over the course of 48-118 weeks.9 In contrast to the other studies discussed so far, treatment failure (defined as first confirmed relapse or permanent study discontinuation) was the primary outcome measure with no difference between teriflunomide 14mg versus IFN-b. For the secondary end-point, ARR, there was no signficant difference between IFN-b and 14mg teriflunomide (0.22 and 0.26 respectively, p=0.60). Other secondary outcomes included fatigue as measured by the Fatigue Impact Scale and patient satisfaction and both were found to be signficantly improved in those allocated to teriflunomide compared to IFN-b.
Safety
Throughout the entire teriflunomide MS development programme (placebo controlled trials and their extensions), a total of 2284 patients have been exposed to teriflunomide at either 7mg or 14mg with a maximum exposure of 11.1 years. There have been 13 deaths; 5 occuring during the placebo-controlled trials and 8 during the extension studies. No evidence indicates teriflunomide as the causative agent.10
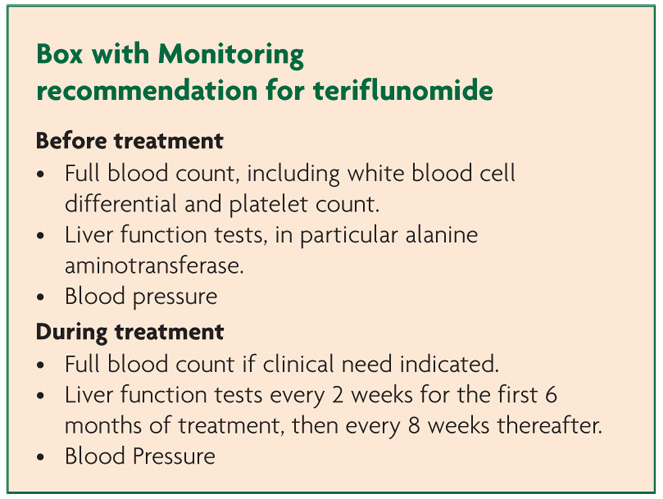
In both TOWER and TEMSO adverse events occurred at a similar rate across the teriflunomide and placebo groups with 82% of those in the placebo group reporting at least one adverse event compared with 84% in the 14mg teriflunomide arm (TOWER).8 The corresponding values in TEMSO were 87.5% and 90.8%.7 Serious adverse events in TOWER occurred at an equal rate with 12% of partipants reporting them in the placebo and 14mg teriflunomide groups.8 A higher rate of serious adverse events were found in those taking teriflunomide in TEMSO with 15.9% compared to 12.8% in placebo.7
The most frequent treatment-emergent adverse events found in a pooled analysis of all the placebo-controlled Phase II/III studies include headache, Diarrhoea, nausea, raised ALT and alopecia.11
Leucopenia (neutropenia and lymphopenia) was found across all studies but this never exceeded 15%, importantly was not correlated with infection and resolved on discontinuation.10 Serious infection occurred in 2.5% of participants in both placebo and 14mg groups with nasopharyngitis accounting for most of the cases. Two serious opportunistic infections were reported in TOWER; a case of intestinal tuberculosis (teriflunomide 14mg) and a case of hepatitis C with cytomegalovirus (placebo).10
Patients taking teriflunomide were more likely to experience rises in blood pressure (both systolic and diastolic measures) particularly affecting those with baseline hypertension indicating need for concomitant blood pressure monitoring.10 The aetiology of this is uncertain as there are no obvious effects of the drug on the kidney, vasoconstriction, increased heart rate or fluid retention. No instances led to study discontinuation.
Peripheral neuropathy was also reported at higher rates within the treatment groups, with 10 cases in TEMSO and 18 in TOWER encompassing both mononeuropathies and polyneuropathies with no reported cases in placebo groups.10 It is however difficult to determine cause and effect within this clinical population group due to the nature of the disease process.
Teriflunomide has been found to be fetotoxic in animal studies. There have been 81 pregnancies throughout the clinical programme with available data on 66.11 Of these, 23 were healthy pregnancies, 12 spontaneous and 26 induced abortions and 5 ongoing pregnancies. There is a long ‘washout’ period, with two drug-free years required before women can conceive. On a related note, consideration to type or dose of oral contraceptive should be used as teriflunomide been found to increase the exposure to ethinylestradiol and levonorgestral.12
Long-term clinical data does not indicate an increase in either haematological and solid tumour malignancies above the population level.13
Adverse events during treatment leading to treatment discontinuation were higher in the teriflunomide arm compared to placebo across both TEMSO (10.9% versus 8.1% respectively) and TOWER (16% versus 6%).7-8 However, overall a greater proportion of patients in the placebo arms discontinued treatment compared to the active arms in both TEMSO (29% versus 27%) with the reverse seen in TOWER (32% versus 34%).7-8 In the active comparator study TENERE, 22% of those on IFN-b discontinued treatment permanently due to adverse events compared to just 12% in the teriflunomide 14mg group. So although data from the clinical trials suggests that IFN-b is clinically equivalent to teriflunomide 14mg, this data suggests that the latter is associated with better tolerance of side effects.
References
- National Institute of Clinical Excellence. Teriflunomide for treating relapsing–remitting multiple sclerosis. 2014. http://publications.nice.org.uk/teriflunomide-for-treating-relapsingremitting-multiple-sclerosis-ta303.
- Strand V, Cohen S, Schiff M, et al. Treatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate. Leflunomide Rheumatoid Arthritis Investigators Group. Arch Intern Med 1999;159(21):2542-50.
- Greene S, Watanabe K, Braatz-Trulson J, Lou L. Inhibition of dihydroorotate dehydrogenase by the immunosuppressive agent leflunomide. Biochem Pharmacol 1995;50(6):861-7.
- Limsakun T, Menguy-Vacheron F. Pharmacokinetics of oral teriflunomide, a novel oral disease-modifying agent under investigation for the treatment of multiple sclerosis. Neurology 2010;74:P05.032.
- Rozman B. Clinical pharmacokinetics of leflunomide. Clin Pharmacokinet 2002;41(6):421-30.
- Drug Approval Package Aubagio (Teriflunomide) tablets. Clinical Pharmacology and Biopharmaceutics Review(s): NDA992; 2011 August 12, 2011.
- O’Connor P, Wolinsky JS,Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 2011;365:1293-303.
- Confavreux C, O’Connor P, Comi G, Freedman MS, Miller AE, Olsson TP, Wolinsky JS, Bagulho T, Delhay JL, Dukovic D, Truffi net P, Kappos L. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo controlled, phase 3 trial.
- Vermersch P, Czlonkowska A, Grimaldi LM, et al. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Mult Scler 2014;20(6):705-16.
- AubagioTM (teriflunomide). Investigator’s brochure. Sanofi, France (2012).
- Leist T, Freedman M, Kappos L, et al. Pooled safety data from three placebo-controlled teriflunomide studies. Mult Scler 2013;19:74 P633
- Sartori A, Carle D, Freedman MS. Teriflunomide: a novel oral treatment for relapsing multiple sclerosis. Expert Opin Pharmacother 2014;15(7):1019-27.
- AubagioTM (teriflunomide). Summary of product characteristics. Sanofi, Paris, France.

