Abstract
The diagnosis of Parkinson’s disease is often not made until the pathology is advanced. The existence of prodromes prior to diagnosis are now well recognised. In this review, we focus on the research attempting to characterise prodromal disease with the ultimate aim of identifying individuals that would benefit from early initiation of neuroprotective therapies.
Why should we predict Parkinson’s disease?
Parkinson’s disease (PD) is the second most common neurodegenerative condition after Alzheimer’s disease, and is associated with increasing age. As population demographics change worldwide, the prevalence and societal burden of PD will grow.1 Currently the diagnosis is made once key motor features of rigidity, tremor and bradykinesia are established, correlating with an approximate loss of half of the neurons within the substantia nigra.2 Thus, in terms of pathology, PD is not diagnosed until an advanced stage of the disease; limiting the potential benefits that might be obtained from neuroprotective therapies. Over the last decade, the existence of a prodromal (or pre-diagnostic) phase of PD has become evident. This may extend up to 20 years prior to diagnosis,3 during which time a variety of non-motor symptoms may emerge. These features include autonomic symptoms such as orthostatic hypotension, bowel, bladder and sexual dysfunction; olfactory dysfunction; sleep disorders such as REM-sleep behaviour disorder (RBD); excessive daytime somnolence; and cognitive and mood changes. This phase is unlikely to be exclusively non-motor, and increasing evidence suggests subtle motor dysfunction is also present at this time.4 Up to 2% of people aged between 65 and 69 years are likely to be in the prodromal phase of PD, with higher proportions at increasing ages.5 A method to identify those at high risk of PD is a vital pre-requisite to enrolment in clinical trials, and subsequently to screen the population and select those likely to gain benefit from preventive or disease-modifying treatments.
How should we predict PD?
Prediction in high-risk cohorts
Several groups have adopted an ‘enrichment’ approach by identifying individuals with a single strong risk factor such as non-manifesting GBA or LRRK2 gene mutation carriers. Here we use an alternative example of patients with RBD confirmed using polysomnography (PSG-RBD). The lifetime risk of individuals with PSG-RBD developing a synucleinopathy (PD, multi-systems atrophy (MSA) or dementia with lewy bodies (DLB)) exceeds 70%.6 Such a high magnitude of risk may justify enrolment into clinical trials without further selection because the proportion that develop parkinsonism is approximately 10% per year.7 However, the application of a secondstage test could decrease the length of trial follow-up required. For example, stratifying an RBD cohort according to the presence of additional prodromal symptoms, such as mild motor dysfunction and impaired olfaction, has been shown to identify subpopulations with a greater than 60% risk of PD development at three years.7 One problem is that patients with idiopathic RBD can be difficult to identify; the disorder is not common and the diagnosis requires an overnight sleep study. PSG is expensive and time-consuming, and may be of limited value in identifying very large cohorts of individuals at risk of PD. It is also likely that RBD-associated parkinsonism represents a variant of the disease with greater cognitive impairment and more autonomic dysfunction, giving rise to a ‘diffuse/malignant’ subtype of PD; characterised by an accelerated decline in motor and non-motor functions.8 If disease-modifying therapeutic success were achieved in these patients, it may not necessarily be replicated in a wider prodromal PD population. An ‘enriched’ population may also be identified through the use of a multi-stage screening process: the primary stage would involve the application of a high-sensitivity ‘test’ to a general elderly population, followed by a second stage test, such as imaging, to achieve greater specificity for prodromal disease detection. Jennings et al. (2017)9 used this strategy to screen a general population cohort for hyposmia with the widely used University of Pennsylvania Smell Identification Test, and hyposmic individuals subsequently underwent (123I)ß-CIT SPECT scans. This method yielded promising results with a high specificity for prodromal PD detection (98%), and relatively good sensitivity (74%) and positive predictive value (PPV, 67%).
Prediction models in the wider population
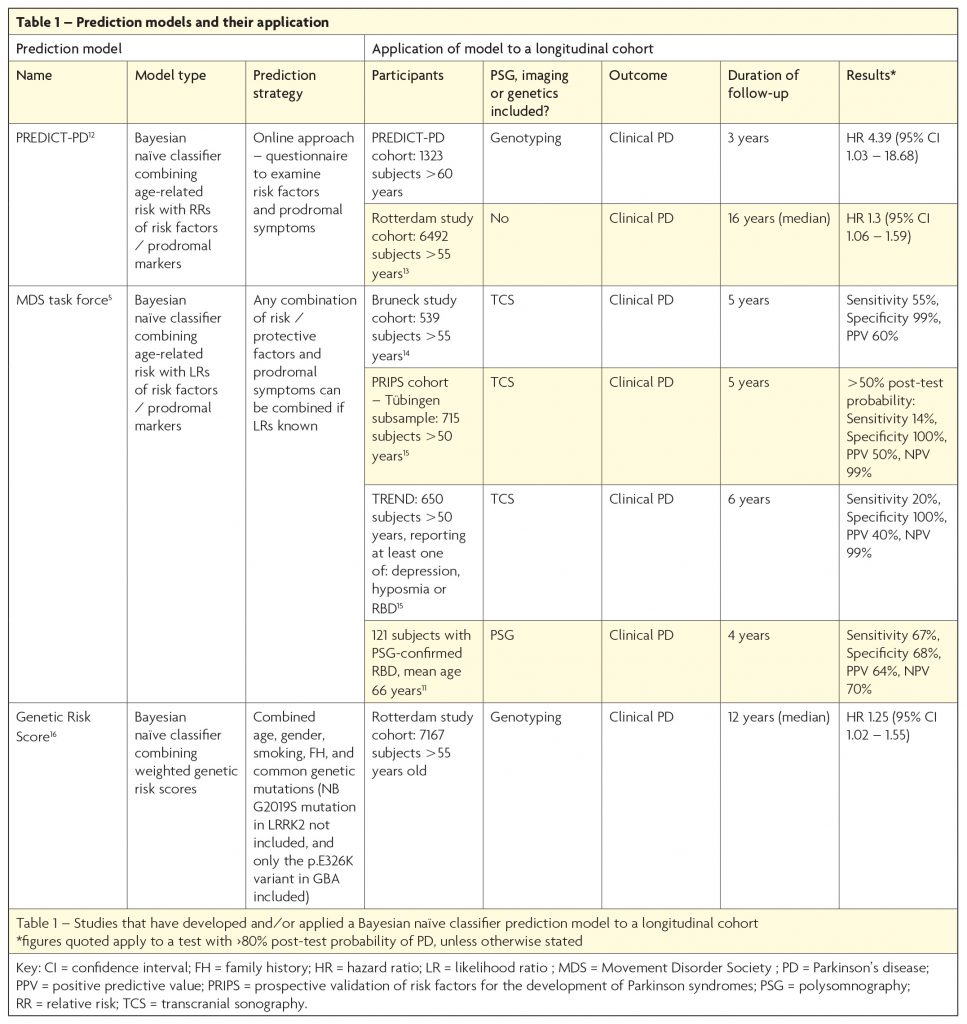
An alternative method is to apply a ‘risk algorithm’ to an unselected elderly population. Such algorithms use a Bayesian naïve classifier approach to add or subtract ‘likelihood ratios’ associated with the presence or absence of multiple risk or protective factors, imaging abnormalities, and/or prodromal symptoms. Several groups have adopted such a method and the results are outlined in Table 1. Interest in prediction models has increased in recent years, with the recognition that they hold certain advantages over the enrichment approach. One benefit of the algorithmic method is that it individualises risk, so that the high-risk cohort includes a range of people with different combinations of prodromal features and risk factors, with or without additional genetic risk markers. The resulting cohort is more likely to be representative of the true prodromes of PD, when compared to a population enriched by the presence of one relatively uncommon high-risk prodromal marker (such as PSG-RBD), or by a fixed combination of prodromal symptoms. Additionally, the flexibility of this approach enables application of the algorithm in a variety of ways, depending upon the information available. This is evidenced by the diversity of cohorts to which the MDS research criteria for prodromal PD have already been applied (see Table 1).
Despite such benefits, the prediction model strategy is not without disadvantages. Essentially a ‘test’ is applied to a population, with a predefined outcome (for instance >80% likelihood of prodromal PD) stating whether an individual is ‘test positive’ or ‘test negative’ for prodromal PD. Applying such a test to an unselected population, with a low background prevalence of PD, naturally leads to lower PPVs than it would if applied to an enriched population. Consequently, use of this approach to enrol people into clinical trials means a high number of trial participants will not go on to develop PD, potentially exposing a substantial number to unnecessary risk. Thus far, only Mahlknecht et al (2016),10 have achieved a relatively high PPV (60%) using this method, by applying the MDS Task Force criteria to the Bruneck study cohort (see Table 1).
Is a combined approach the best way forward?
The two broad strategies described are not mutually exclusive, and indeed have already been combined in one recent study where the MDS criteria were applied to a PSG-RBD cohort.11 As expected, the application of a prediction model to an enriched population, with a high pre-test probability of PD, led to a relatively high PPV of 64% at four years (see Table 1), which increased to 100% when follow-up duration was extended to ≥10 years. Though this approach is hindered by the aforementioned limitations of using a PSG-RBD cohort, it emphasises the importance of the review duration of follow-up when making an accurate determination of the sensitivity, specificity, PPV and NPV of a prodromal disease ‘test’. Perhaps the most promising strategy for the future is to use a two-stage process which combines both approaches: use of an algorithm to screen for high-risk individuals in the general population as the first step (with or without a genetic risk score), before applying a second stage test using quantitative, objective assessments such as smell tests, motor assessments and/or imaging, such as DAT-deficit on (123I) ß-CIT SPECT or substantia nigra hyperechogenicity on transcranial sonography. This is the approach adopted by the PREDICT-PD study, which uses an online strategy to screen for those at high-risk, before inviting selected participants for further investigation.12
Conclusion
The field of prodromal PD research has advanced considerably over the last decade, and we are now at the stage where high-risk cohorts are being assembled for clinical trial enrolment. The development of prediction models offers the advantage of greater flexibility and generalisability than alternative approaches. However, utility is dependent upon the information available, and higher positive predictive values may come at the cost of reduced feasibility. Work remains in order to refine prediction methods, in parallel with identifying robust disease biomarkers.
References
- Dorsey ER, Bloem BR. The Parkinson Pandemic—A Call to Action. JAMA Neurology. 2017.
- Fearnley JM, Lees, AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114:2283-301.
- Hawkes CH, Del Tredici K. Braak, H. (2010). A timeline for Parkinson’s disease. Parkinsonism & Related Disorders. 2010;16:79–84.
- Postuma RB, Lang AE, Gagnon JF, Pelletier A, Montplaisir JY. How does parkinsonism start? Prodromal parkinsonism motor changes in idiopathic REM sleep behaviour disorder. Brain 2012;135:1860-70.
- Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, Gasser T, Goetz CG, Halliday G, Joseph L et al. MDS research criteria for prodromal Parkinson’s disease. Movement Disorders 2015;30:1600-11.
- Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 2013;14:744-8.
- Postuma RB, Gagnon JF, Bertrand JA, Génier Marchand D, Montplaisir JY. Parkinson risk in idiopathic REM sleep behavior disorder: preparing for neuroprotective trials. Neurology 2015;84:1104-13.
- Fereshtehnejad SM, Romenets SR, Anang JBM, Latreille V, Gagnon JF, Postuma RB. New Clinical Subtypes of Parkinson Disease and Their Longitudinal Progression: A Prospective Cohort Comparison With Other Phenotypes. JAMA Neurol 2015;72,:863-73.
- Jennings D, Siderowf A, Stern M, Seibyl J, Eberly S, Oakes D, Marek K, and for the PARS Investigators. Conversion to Parkinson Disease in the PARS Hyposmic and Dopamine Transporter–Deficit Prodromal Cohort. JAMA Neurology 2017;74:933.
- Mahlknecht P, Gasperi A, Willeit P, Kiechl S, Stockner H, Willeit J, Rungger G, Sawires M, Nocker M, Rastner V, et al. Prodromal Parkinson’s disease as defined per MDS research criteria in the general elderly community. Mov Disord. 2016; 31:1405-08.
- Fereshtehnejad SM, Montplaisir JY, Pelletier A, Gagnon JF, Berg D, Postuma RB. Validation of the MDS research criteria for prodromal Parkinson’s disease: Longitudinal assessment in a REM sleep behavior disorder (RBD) cohort: Validation of the MDS Prodromal Parkinson Research Criteria. Movement Disorders 2017;32:865-73.
- Noyce, AJ, R’Bibo L, Peress L, Bestwick JP, Adams-Carr KL, Mencacci N, Hawkes CH, Masters JM, Wood N, Hardy J, et al. PREDICT-PD: An online approach to prospectively identify risk indicators of Parkinson’s disease. Movement Disorders 2017:32;219-26.
- Darweesh SKL, Koudstaal PJ, Stricker BH, Hofman A, Steyerberg EW, Ikram MA. Predicting Parkinson disease in the community using a nonmotor risk score. Eur. J. Epidemiol. 2016a:31;679-84.
- Mahlknecht P, Gasperi A, Willeit P, Kiechl S, Stockner H, Willeit J, Rungger G, Sawires M, Nocker M, Rastner V, et al. Prodromal Parkinson’s disease as defined per MDS research criteria in the general elderly community. Mov Disord. 2016:31;1405-08.
- Pilotto A, Heinzel S, Suenkel U, Lerch, S, Brockmann K, Roeben B, Schaeffer E, Wurster I, Yilmaz R, Liepelt-Scarfone I, et al. Application of the movement disorder society prodromal Parkinson’s disease research criteria in 2 independent prospective cohorts: Application of Research Criteria For Prodromal PD. Movement Disorders 2017:32;1025-34.
- Darweesh SKL, Verlinden VJA, Adams HHH, Uitterlinden AG, Hofman A, Stricker BH, van Duijn CM, Koudstaal PJ, Ikram MA. Genetic risk of Parkinson’s disease in the general population. Parkinsonism & Related Disorders 2016b:29;54–59.

