Summary
- Visual misperceptions and hallucinations are common in Parkinson’s disease and exist along a spectrum.
- Recent work has suggested that visual misperceptions may be related to deficits in attention, rather than pure disorders of perception.
- Results from multimodal neuroimaging studies have provided strong supportive evidence for the hypothesis that visual misperceptions are due to abnormal communication within and between Attentional Control Networks.
- The mechanisms underlying visual misperceptions in Parkinson’s disease provide a novel framework for understanding hallcuinatory phenomena in other neurobiological disorders.
Visual misperceptions and hallucinations (VH) occur in over half of all patients with Parkinson’s disease (PD), particularly in the latter stages of the condition.1 These symptoms often occur along a distinct spectrum, with visual misperceptions representing the incorrect recognition of a perceived stimulus and hallucinations representing the occurrence of a perception in the absence of a clear stimulus. Despite their negative impact on patient outcomes,2 these neuropsychiatric symptoms remain poorly understood in PD, with limited therapeutic options.1
Although the pathophysiology of VH is poorly understood, a number of theories have been proposed as explanations for VH in PD. For instance, the presence of VH in clinical disorders that affect the retina, such as Charles Bonnet Syndrome,3 has led to the proposal that the VH suffered in PD may result from impairments within the visual pathway. In keeping with this hypothesis, it is well recognised in clinical practice that reduced ambient lighting and unfamiliar environments are associated with increased perceptual errors. This has led to the proposal that these visual impairments may induce a partial sensory deprivation that permits the emergence of previously recorded percepts, which then form the basis for VH.4
Despite the visual impairments present in hallucinators, it has become increasingly clear these same patients also suffer from specific deficits in executive function and attention. For example, a previous fMRI study has demonstrated that PD patients with chronic VH respond to the presentation of simple visual stimuli with greater frontal and caudate nucleus activation and less visual cortical activation than non-hallucinating PD subjects.5 In addition, a strong clinicopathological correlation has been found between the presence of VH and Lewy body (LB) pathology within temporal cortical structures, such as the amygdala and parahippocampal gyrus,6 suggesting that VH in PD relate to a disruption across diverse neural circuitry involved with attention and perception.
These findings have led to several proposals suggesting that hallucinations in PD are related to deficits in perception and attention,7,8 perhaps due to both modulatory neurotransmitter disturbances as well as specific subcortical and cortical pathology.6 More recently, combining these insights with findings from the broader neuroimaging literature, it has been proposed that visual misperceptions and hallucinations in PD are due to specific communication breakdowns in the connectivity between the large-scale neuronal networks that subserve attention and conscious perception.
Known as the Attentional Control Networks, these comprise the Dorsal Attention Network (DAN), the Ventral Attention Network (VAN) and Default Mode Network (DMN),9 each of which is responsible for unique aspects of visual attention (see Table 1). For example, the DAN is consistently activated during tasks that require exogenous attention (e.g. using saccadic eye movements to track an external object), whereas the DMN is more regularly associated with internally focused tasks (e.g. episodic memory recall and internal mentation) (see Table 1). The VAN is presumed to be responsible for the more domain-general function of ‘salience monitoring’ and also mediates the switch between the DAN and the DMN. Coordinated activity between these networks has been shown to underlie a number of more complex attentional tasks, such as goal-directed episodic recall.10
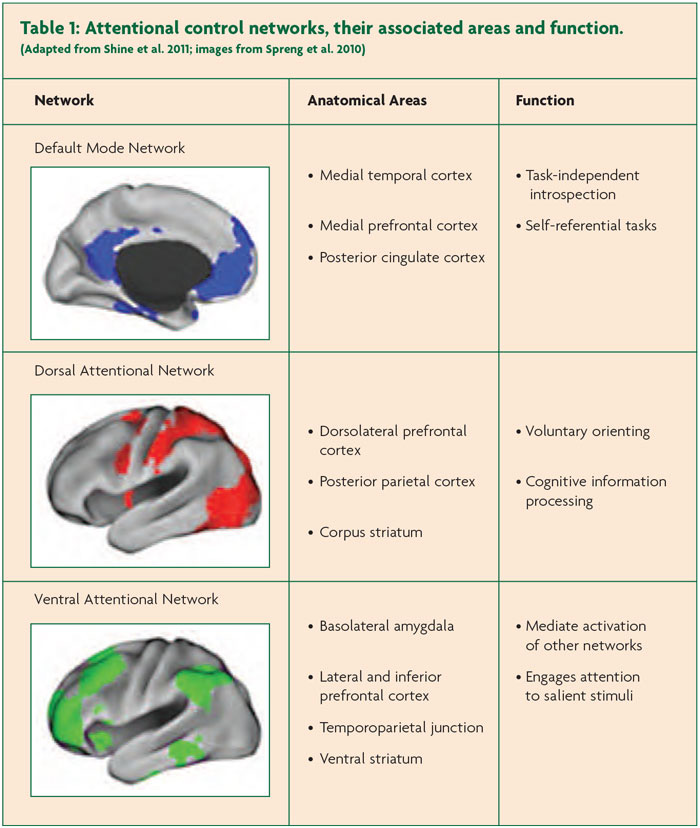
Specifically, the Attentional Networks Hypothesis proposes that VH in PD are due to a relative inability to recruit activation in the DAN in the presence of an ambiguous percept, leading to an ‘over reliance’ on the VAN and DMN. The DAN, which underlies the capacity to focus attention on externally driven percepts, is comprised of widespread neural regions within the frontal eye fields, the dorsolateral prefrontal cortex and the superior posterior parietal cortices, all of which send efferents to the head of the caudate nucleus.11 The model proposes that an inability to recruit this network would lead to an over-reliance on the VAN and the DMN, both of which are poorly suited to interpret the content of ambiguous sensory images.10
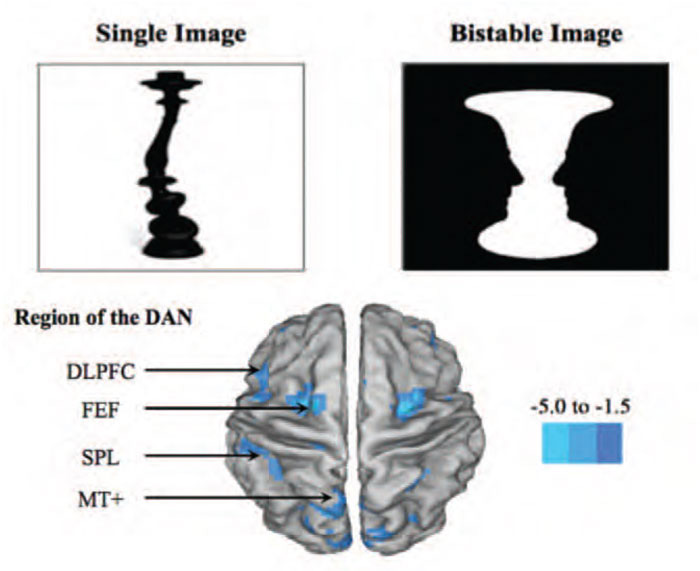
The attentional network hypothesis of VH has received preliminary supportive evidence through behavioural testing using the novel Bistable Percept Paradigm (BPP;9). The BPP is a computer-based task that requires participants to evaluate a battery of monochromatic ‘monostable’ and ‘bistable’ percepts (see Figure 1) and determine whether there are any images ‘hidden’ within each picture (e.g. the silhouettes of faces on the edges of the vase in Figure 1). Although the task appears relatively straightforward, performance on the task is able to dissociate patients that hallucinate from those without the symptom. For example, in a cohort of 45 patients with PD, the 23 patients that performed poorly on the BPP were significantly more likely to self-report hallucinations and also displayed specific impairments in attentional set-shifting ability, implicating an inability to effectively recruit the DAN in PD patients with VH.12
Impaired performance on the BPP has also been shown to be predictive of specific neuronal impairments in patients with PD. Using Magnetic Resonance Spectroscopy (or MRS), which is a neuroimaging technique that allows for the estimation of neuronal integrity in specific regions of the brain through the exploration of their spectroscopic signature, in a cohort of 22 patients with PD, a recent study found that the severity of impairments on the BPP were associated with reduced neuronal integrity (as measured by the ratio of N-Acetyl Cysteine to Creatine) in the anterior cingulate cortex, a neural hub that connects the DAN and the VAN and is implicated in decision making and conflict monitoring.13
In addition to this spectroscopic data, a recent multi-modal neuroimaging study has explored the neural correlates of performance on the BPP in relation to the Attentional Network Hypothesis.14 In this study, 22 patients with PD performed the BPP in an fMRI scanner while ‘on’ their regular dopaminergic medications. Hallucinators who had poor performance on the BPP showed significantly decreased activity in their BOLD signal within the DAN in keeping with the major prediction of the Attentional Network model.14 In addition, structural and resting-state data allowed for the interrogation of grey matter integrity and relative “connectivity” during the brain’s “idling” rhythm, respectively. Analysis of the structural brain scans showed that hallucinators had a relative loss of grey matter volume within the anterior insula, a key hub within the VAN that facilitates the shifting of attention to the DAN in the presence of ambiguous visual stimuli. Furthermore, an exploration of the resting-state connectivity within the attentional control networks revealed reduced coherence between the DAN and the VAN amongst hallucinators. Together, the findings from these three complementary analyses were all strongly and predictably inter-related, showing high degrees of inter-correlation, strengthening the notion that each arm of the study was providing insight into a unique aspect of the pathophysiology underlying visual misperceptions and hallucinations.
In conclusion, these results provide support for the notion that VH in PD are due to a breakdown in communication between large-scale neuronal networks that sub-serve attentional mechanisms. Interestingly, the mechanism described above may indeed provide a novel neurobiological framework underlying hallucinations, regardless of the specific disorder in which they occur. For example, extension of these principles to the auditory domain may help explain some of the core features within Schizophrenia with the proposed pathophysiological model operating across similarly disturbed networks. It is hoped that using this novel framework may help to provide the basis for integrated research across disciplines facilitating novel therapeutic strategies.
References
- Diederich NJ, Fenelon G, Stebbins G. & Goetz CG. Hallucinations in Parkinson disease. Nature Reviews Neurology 2009;5:331-42.
- Goetz CG, Fan W, Leurgans S, Bernard B & Stebbins GT. The malignant course of ‘benign hallucinations’ in Parkinson disease. Arch. Neurol. 2006;63:713-16.
- Ffytche DH. Visual hallucinations and the Charles Bonnet syndrome. Curr Psychiatry Rep. 2005;7(3):168:79.
- Diederich NJ, et al. Poor visual discrimination and visual hallucinations in Parkinson’s disease. Clin Neuropharmacol 1998;21:289-95.
- Stebbins GT, et al. Altered cortical visual processing in PD with hallucinations: an fMRI study. Neurology 2004;63:1409-16.
- Harding AJ, Broe GA & Halliday GM. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain 2002;125:391-403.
- Collerton D, Perry E & McKeith I. Why people see things that are not there: A novel Perception and Attention Deficit model for recurrent complex visual hallucinations. 2005:1-58.
- Diederich NJ, Goetz CG & Stebbins GT. Repeated visual hallucinations in Parkinson’s disease as disturbed external/internal perceptions: Focused review and a new integrative model. Mov Disord. 2005;20:130-40.
- Shine JM, Halliday GM, Naismith SL & Lewis SJG. Visual misperceptions and hallucinations in Parkinson’s disease: Dysfunction of attentional control networks? Mov Disord. 2011;26:2154-9.
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW & Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage 2010;53:303-17.
- Asplund CL, Todd JJ, Snyder AP & Marois R. A central role for the lateral prefrontal cortex in goal-directed and stimulus-driven attention. Nature Publishing Group 2010;13:507-12.
- Shine JM, Halliday GH, Carlos M, Naismith SL & Lewis, SJG. Investigating visual misperceptions in Parkinson’s disease: a novel behavioral paradigm. Mov Disord. 2012;27:500-5.
- Lewis SJG, Shine JM, Duffy S, Halliday G & Naismith SL. Anterior cingulate integrity: Executive and neuropsychiatric features in Parkinson’s disease. Mov Disord. 2012;27:1262-7.
- Shine JM, et al. The role of dysfunctional attentional control networks in visual misperceptions in Parkinson’s disease. Hum Brain Mapp. 2013; EPub Ahead of Print.

