A female patient was diagnosed with sensorineural deafness aged 37. She then presented at the age of 45 years with a progressive cerebellar syndrome. Blood DNA analysis detected a nucleotide 3243 A–>G mutation in the mitochondrial DNA confirming a diagnosis of Mitochondrial Encephalomyopathy with Lactic Acidosis and Stroke-like episodes (MELAS). At the age of 48 she presented with focal neurological deficits, encephalopathy and seizures. She was treated with sodium valproate with resultant development of partial status. Seizure control was gained following withdrawal of valproate and establishment of phenobarbitone. This case illustrates that valproate should be avoided in the treatment of complex partial status epilepticus (CPSE) when it occurs in the context of MELAS.
Clinical synopsis
At the age of 45 years, a female shop assistant presented with slurred speech, tremor and unsteady gait. Examination revealed nystagmus, dysarthria, gait and limb ataxia.Her past medical history was of sensorineural deafness, diagnosed in 1996.There was no relevant family history.Neuroimaging was normal.She went on to have further investigations and blood leucocyte DNA analysis revealed an A-to-G substitution at nucleotide position 3243 of the mitochondrial DNA.This confirmed the suspected diagnosis of MELAS.
The patient originally presented with a severe left-sided headache, visual impairment and low grade pyrexia. She had two focal (frontal) seizures with secondary generalisation and was treated with intravenous phenytoin. Brain computer tomography and lumbar puncture were normal. She was treated empirically for a meningo-encephalitis with intravenous acyclovir and ceftriaxone. Magnetic Resonance Imaging of the brain revealed a lesion in the left occipital lobe (Figure 1).
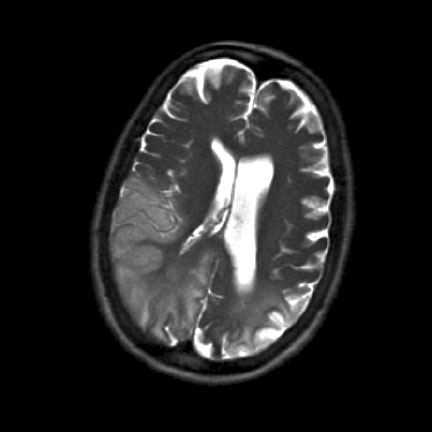
Despite adequate loading with phenytoin she continued to have seizures. She also developed a florid rash which was attributed to the phenytoin. Therefore she was started on intravenous sodium valproate at a dose of 500 mg twice daily and the phenytoin was discontinued. The rash resolved completely over the course of the next 24 hours. However, seizure control worsened and she developed CPSE. Oral phenobarbitone was added and sodium valproate was withdrawn with subsequent establishment of seizure control. An electroencephalogram showed slowing of the alpha rhythm and frequent epileptiform discharges. She was started on treatment with L-Arginine and Co-enzyme Q10 and had rehabilitation therapy. Her clinical condition gradually improved. On discharge she had a left homonymous hemianopia, dysphasia, left-sided hemi-neglect and ataxia.
Discussion
There is a presumed link between valproate and the exacerbation of seizures in patients with MELAS based on 3 previous case reports1,2,3. There is no clear mechanism behind this effect although it is not an isolated phenomenon as there are other mitochondrial diseases in which valproate use has adverse effects. Patients with mitochondrial polymerase gamma 1 (POLG1) gene are susceptible to hepatic failure and encephalopathy which appears to be exacerbated by the use of valproate4. Defects in the POLG1 gene produces syndromes with a high degree of phenotypic variability – ranging from Alpers syndrome to progressive external ophthalmoplegia. A POLG1 defect has also been isolated in a patient with a syndrome which fulfilled all the diagnostic criteria for MELAS5 suggesting that there may be some overlap between these two groups. A recent case series reported 2 out of 19 patients with the POLG1 mutation developing hepatic failure after valproate exposure6.
Oral supplementation of Coenzyme Q10 has been suggested as a possible treatment for the myopathy associated with MELAS as patients are found to have low circulating serum levels – although it is of note that muscle levels are normal. Supplementation has been found to be of benefit in individual cases although this has not been supported in larger trials and there is no clear mechanism for its positive effects7. Despite the lack of evidence a trial of Coenzyme Q10 is still advocated as it may produce some improvement in the degree of myopathy7,8. Two cases have been reported in the literature which relate statin use to precipitating symptoms in patients with MELAS9,10. The mechanism is thought to be due to statins inhibiting Coenzyme Q10 as a part of their action and worsening a pre-existing lack of this cofactor in susceptible patients which further lends support to the argument for Coenzyme Q10 supplementation.
The supplementation of L-Arginine has also been advocated in patients with MELAS. Concentration of L-Arginine during an acute exacerbation was found to be significantly lower than in control subjects. Intravenous infusion of L-Arginine led to clinical improvement in patients presenting with a stroke-like episode within 30 minutes and two years of oral supplementation significantly decreased both frequency and severity of stroke-like episodes11. However, controlled trials still need to be carried out to confirm the effectiveness of L-Arginine as a therapy and to evaluate any adverse effects.
There has been much debate about the pathogenesis of the stroke-like episodes in MELAS. The lesions often occur in the occipital lobe and do not adhere to a typical arterial distribution/vascular territory. Current opinion supports a metabolic rather than an ischaemic cause for the lesions. Diffusion-weighted magnetic resonance imaging (DWI) is the imaging modality of choice in patients with suspected MELAS (demonstrating normal or increased apparent diffusion coefficient values). In one study enrolling 12 patients with MELAS, DWI was found to be more sensitive than conventional MRI12.
Conclusion
Since there is some evidence that sodium valproate leads to seizure exacerbation in MELAS, it should be avoided in patients with this condition and alternative anti epileptic therapy used. Both L-Arginine and Coenzyme Q10 have a role in the management of these patients, but larger studies are needed to confirm the benefit. DWI is the imaging modality of choice in patients with suspected MELAS.
References
- Lam CW, Lau CH, Williams JC, Chan YW, Wong LJ. Mitochondrial Myopathy, Encephalopathy, Lactic Acidosis and Stroke- like Episodes (MELAS) Triggered by Valproate Therapy. Eur J Pediatrics 1997;156(7):562-564.
- Lin, CM, Thajeb, P. Valproic Acid Aggravates Epilepsy due to MELAS in a Patient With an A3243G Mutation of Mitochondrial DNA. Met Brain Dis 2007;22(1):105-109.
- Hsu YC, Yang FC, Perng CL, Tso AC, Wong LJ, Hsu CH: Adult Onset of Mitochondrial Myopathy, Encephalopathy, Lactic Acidosis and Stroke Like Episodes (MELAS) Syndrome Presenting as Acute Meningoencephalitis: A Case Report. J Emerg Med 2009;0736-4679.
- Horvath et al Phenotypic Spectrum Associated with Mutations of the Mitochondrial Polymerase Gamma Gene. Brain 2006;129(7):1685-92.
- Deschaeur M, Tennant S, Rokicka A, He L, Kraya T, Turnbull DM, Zierz S, Taylor RW. MELAS Associated with Mutations in the POLG1 Gene. Neurology 2007;15:68(20):1741-2.
- Engelson BA, Tzoulis C, Karlsen B, Lillebo A, Laergreid LM, Aasly J, Zeviani M, Bindoff LA. POLG1 Mutations Cause a Syndromic Epilepsy with Occipital Lobe Predilection. Brain 2008;131(3):818-28.
- Berbel- Garcia A, Barbera- Farre JR, Etessam JP, Salio AM, Cabello A, Gutierrez- Rivas E, Campos Y. Coenzyme Q 10 Improves Lactic Acidosis, Stroke Like Episodes, and Epilepsy in a Patient with MELAS (Mitochondrial Myopathy, Encephalopathy, Lactic Acidosis, and Stroke Like Episodes). Clin Neuropharmacol 2004; 27(4):187- 191.
- Goda S, Hamada T, Ishimoto S, Kobayashi T, Goto I, Kuroiwa Y. Clinical Improvement After Administration of Coenzyme Q10 in a Patient with Mitochondrial Encephalomyopathy. J Neurol 1987;234(1):62-3.
- Thomas JE, Lee N, Thompson PD. Statins Provoking MELAS Syndrome. A Case Report. Eur Neurol 2007;57(4):232-5.
- Chariot P, Abadia R, Danan C, Charpentier C, Gherardi RK. Simvastatin-induced Rhabdomyolysis Followed by a MELAS Syndrome. Am J of Med 1993;94(1)109-10: 0002-9343
- Koga Y, Akita Y, Nishioka J, Yatsuga S, Povalko N, Katayama K, Matsuishi T. MELAS and L-Arginine therapy. Mitochondrion 2007;7:133- 139.
- Abe K, Yoshimura H, Tanaka H, Fujita N, Hikita T, Sakoda S: Comparison of Conventional and Diffusion- Weighted MRI and Proton MR Spectroscopy in Patients with Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke Like Events. Neuroradiology 2004;46(2):113- 117.


