Introduction by Jeremy Chataway
Welcome to this new series in ACNR. The first two articles focus on two aspects of multiple sclerosis (MS). As you will know, nearly 3M globally and around 135,000 in the UK are affected, with large societal, healthcare and individual costs. In the last three decades there have been enormous advances with the development of the disease modifying treatments (DMT), especially for relapsing disease. Depending on how they are categorised, the number now touches 20 with a variety of mechanisms of action.
Yet despite this good news, the majority of the therapeutic effect comes from immunomodulation, and traction while the neurodegenerative aspects has been much less. Whilst there is much phase 2 and phase 3 activity [1], this complex progressive biology remains the cardinal problem, and indeed is likely to start from a very early stage in the disease.
It is well described how a variety of co-morbidities drive disability accumulation in MS, and indeed compared to complex DMT prescription, their attenuation is relatively simple in medical terms, for example, thorough treatment of anxiety and depression (prevalence around 20%). In this issue Charles Wade takes us through the epidemiology of vascular co-morbidities in particular, the effect sizes and how these can be treated to target, using well described risk calculators. There is no doubt they are under-treated and yet the tools exist already. We hope that this opportunity will be made explicit in both primary and secondary care situations, rather than awaiting untreated natural vascular history. The article ends with again the highly modifiable situation of osteoporosis, which in this population, for a variety of reasons, has a higher prevalence than the general population. Again, relatively simple to treat.
Whilst the role of the neurologist may not be to prescribe the losartan or the alendronate, our role is to be aware of these issues, flag them up appropriately, and ensure that they are looked for and treated to target, to avoid later downstream effects. The article contains flow chart summaries of current NICE guidelines for the management of these comorbidities.
In the next issue we have taken the opportunity to summarise current DMT options in progressive MS (PMS). Sean Apap Mangion shows us the evidence, rationale, criteria and risk profile of the two main classes of DMT: siponimod (secondary progressive MS) and ocrelizumab (primary progressive MS). Of particular interest, and a need for some vigilance, is the use of these classes of drugs in a relatively older population – which of course tends to be those with PMS. Issues such as hypertension with siponimod, and an increased risk of viral infection more generally (for example, HSV1 and VZV) are well described. The balance of effectiveness and side-effects needs constant evaluation in the face of chronic treatment. A number of prospective observational cohorts are active and will report over the next few years to further guide our decision making. These will complement a number of phase 3 clinical trials with new compounds such as the Bruton tyrosine kinase inhibitors, which have the potential to act more centrally in the nervous system, and will start to read out in the next 1-2 years.
I hope you enjoy these two articles and they provide useful practical information to make the lives of those suffering from MS, whom you look after, just a little better.
- Chataway J, Williams T, Li V, Marrie RA, Ontaneda D, Fox RJ. Clinical trials for progressive multiple sclerosis: progress, new lessons learned, and remaining challenges. Lancet Neurology. 2024 Mar 1;23(3):277-301.
Comorbidity in the multiple sclerosis clinic
Abstract
Comorbid conditions are common in people with multiple sclerosis (MS) and can lead to diagnostic delay and poorer outcomes. Neurologists have an opportunity to identify and address comorbidities within routine care, without major time or resource burden. This review discusses modifiable comorbidities in MS – focusing on hypertension, dyslipidaemia, diabetes, and osteoporosis highlighting their impact and potential intervention.
Introduction
Multiple sclerosis (MS) is an immune-mediated inflammatory, neuro-degenerative disease of the central nervous system (CNS) [1]. Comorbidity refers to the presence of more than one disease or condition in a person at the same time, where these additional diseases are not directly related complications of the primary disease. They are common in people with MS (PwMS), increasing with age, and are likely contributors to disability [2].
The reported prevalence of comorbidities in PwMS varies widely depending on the study, the range of comorbid conditions considered, the geographic region, the socioeconomic status, the MS type and disease history, and factors such as sex, age, and race [3–8]. Though the prevalence of comorbidities in PwMS could be overestimated when compared to the general population due to higher healthcare utilisation, our understanding of their impact is growing [9]. Emerging evidence has shown that the presence of comorbidities in PwMS increases diagnostic delays, prevents enrolment in clinical trials, impacts disease-modifying therapy (DMT) selection and initiation, increases relapse rates and the rate of disability progression, reduces quality of life, increases rates of hospitalisation, and increases mortality [10–18].
Neurologists and the MS multi-disciplinary team (including nurses, pharmacists, and therapists) have continuous, long-term relationships with their patients and will routinely carry out health assessments (clinical examinations, or screening bloods etc) as part of face-to-face appointments, when initiating or monitoring DMTs, or as part of research work. Given the mounting pressures on general practitioners (GPs), there is an opportunity to identify and, when necessary, address modifiable comorbidities within the neurology clinic setting. We hope to show the importance of weaving this into routine MS care, and that this can be done without taking away significant time or resources.
The primary objective of this review is to discuss prevalent modifiable, comorbid disease in PwMS – specifically looking at hypertension, dyslipidemia, diabetes, and osteoporosis. This list is not exhaustive, and other physical risk factors (including body mass index) are not considered here. The impact of smoking and smoking cessation is also beyond the scope of this review but is discussed extensively elsewhere [19]. It is important to also note that this discussion also does not encompass psychiatric disorders, in particular depression and anxiety (with prevalence of up to 35-40% respectively), which are equally as important and potentially treatable [20].
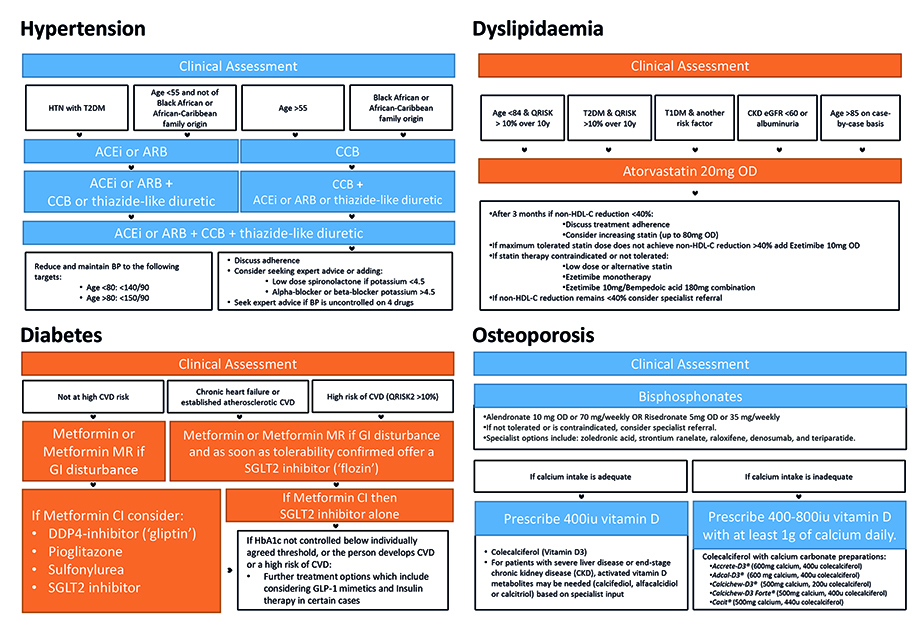
Hypertension
Hypertension has an estimated prevalence of 16-30% in PwMS [3,21-24]. Recent research suggests that hypertension is 25% more common in the MS population compared to non-MS individuals, irrespective of sex and race, and ranks as the third most prevalent comorbidity in MS [25].
Hypertension is a recognised risk factor for numerous disorders, including stroke, coronary artery disease, renal disease, and cognitive decline, all of which can adversely affect ambulatory status, exercise tolerance, and activities of daily living independent of MS. Hypertension is one of the five leading causes of disability in the general population [26]. Several studies suggest hypertension may potentiate brain atrophy, which is particularly relevant in PwMS [27,28].
In MS, hypertension negatively impacts cognitive performance, psychiatric symptoms, progression of visual disability, and progression of lower limb disability [14,29,30]. Furthermore, within the MS population, hypertension is associated with increased mortality risk (though the magnitude of the impact was lower in the MS population than in the matched population) [18].
According to NICE guidance, all adults should have their blood pressure measured at least every five years up to the age of 80 years, and at least annually thereafter. Our recommendation – given the increased prevalence of hypertension in PwMS and the impact it has on their disease – is that more frequent monitoring is sensible. This can be integrated into routine care in most clinical settings, including the face-to-face neurology clinic, DMT monitoring or trial appointment. If the initial clinic BP reading is 140/90 mmHg or higher, the GP can be asked to organise ambulatory blood pressure monitoring (ABPM) or home blood pressure monitoring (HBPM) to confirm the diagnosis. Hypertension is diagnosed if the ABPM or average HBPM is 135/85 mmHg or higher [31].
Many health behaviours can be influenced by brief provider advice embedded within an existing visit for MS care [32]. NICE guidance recommends that managing hypertension starts with education and counselling on lifestyle modifications including weight loss, a healthy diet, reduced alcohol and sodium intake, increased physical activity, and smoking cessation [31]. Regarding diet, evidence bases are now emerging for both the Dietary Approaches to Stop Hypertension (DASH) diet and Mediterranean diet [33,34]. Neurologists can use routine outpatient appointments to ensure that this advice is reiterated and contextualised to MS care just as we have done with smoking and alcohol cessation advice.
In terms of further treatment, research indicates that managing hypertension is not made more difficult by the presence of MS [35]. If the BP remains uncontrolled or the individual is at higher risk of cardiovascular disease, the GP will consider initiating pharmacological treatment, typically starting with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) for those under age 55 years or a calcium channel blocker for those aged 55 and over or of African or Caribbean descent. If necessary, additional medications, such as thiazide-like diuretics or beta-blockers, are added to achieve optimal blood pressure control (Figure 1). The neurologist’s role here would be only to stress the importance of medication adherence.
Dyslipidaemia
The NARCOMS study demonstrated that 37% of the PwMS suffered from hypercholesterolaemia, which was higher than the general population. Though this figure is not consistently repeated in the literature, the incidence rates appear to be rising [22,36-38]. Dyslipidaemia is an independent risk factor for various adverse outcomes associated with disability and even death including stroke, cardiovascular events, peripheral artery disease, kidney disease and vascular dementia. In MS, higher levels of LDL, total cholesterol, and triglycerides are associated with worsening disability, increased relapse rates, impaired overall cognitive function, higher T2 lesion volume, increased brain atrophy, and increased mortality [39-44]. Conversely, higher HDL levels are associated with lower levels of acute inflammatory activity on MRI [39].
A working hypothesis is that the pro-inflammatory and thrombogenic processes associated with dyslipidaemia could plausibly contribute to disease progression in MS via diverse mechanisms at the blood-brain barrier vascular endothelium [45]. This is the basis of the MS-STAT2 trial (ClinicalTrials.gov Identifier NCT03387670) which aims to investigate whether simvastatin, a cholesterol-lowering drug, can slow down the progression of disability in people with SPMS [46]. The hypothesis of the STAT2 trial is that via these pathways, simvastatin has neuro/vasculo-protective properties that could delay disability progression in people with SPMS [47]. This hypothesis is based on previous research, including the phase 2 MS-STAT trial which showed a 43% reduction in atrophy rate compared to control with 80mg/day of simvastatin [48].
NICE guidelines recommend measuring total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides every five years in adults aged 40-74 years. This should likely be more often in PwMS, where despite increased prevalence of dyslipidaemia in PwMS, there is poorer treatment [8,49]. Risk assessment tools, such as QRISK3, are then used to determine the likelihood of developing cardiovascular disease (CVD) within the next 10 years, and treatment recommendations are based on this risk assessment [50]. Management begins with education and lifestyle modifications and for the neurologist, these will be very similar to those advised for hypertension. For individuals at increased risk of cardiovascular disease, the recommendation would be pharmacological treatment with statins. The treatment goal is to reduce non-HDL cholesterol by at least 40% from baseline, and if this is not achieved, the statin dose may be increased, or other lipid-lowering medications, such as ezetimibe may be added (Figure 1) [49].
Diabetes
The focus of this section will largely be Type 2 diabetes mellitus (T2DM) rather than Type 1 (T1DM). While previous studies have shown that T1DM and MS share common immune pathogenetic mechanisms, T1DM is usually detected early in life and thus detection or counselling in the neurology clinic is less likely.
The global incidence of T2DM is on the rise, and PwMS are not spared, with one trial suggesting that T2DM rates are rising faster in the MS population than in an aged-matched general population [3,37]. There appears to be a moderate but significant association of T2DM with MS incidence [51]. Though some of this may reflect increased T2DM diagnosis ascertainment due to higher healthcare utilisation by PwMS following diagnosis, in a recent study, PwMS already had a 30% increased prevalence of T2DM at the time of MS diagnosis when compared to matched controls [8,37].
Diabetes is of course an independent risk factor for disability, not only via nervous system impairment (which will affect up to 70% of those with diabetes), but also by contributing to various chronic conditions such as heart disease and stroke [52]. In MS, diabetes is also known to potentiate disability. A study from Italy using multiple regression analyses revealed that diabetes mellitus was associated with significant reductions in whole brain, grey matter and cortical grey matter volumes in PwMS, and further studies have shown that the presence of comorbidities including diabetes is associated with cognitive dysfunction in MS [42,53].
Treating diabetes likely produces benefits outside lower HbA1c alone. A study by Negrotto et al. investigated the effect of oral antidiabetic medications on paraclinical outcome measures in 50 obese PwMS with metabolic syndrome. The study found that patients receiving metformin hydrochloride and pioglitazone hydrochloride had significantly fewer new or enlarging T2 lesions or gadolinium-enhancing lesions confirmed by brain magnetic resonance imaging after two years of treatment compared to a control group of PwMS with metabolic syndrome who did not receive these medications. Metformin targets Mitochondrial respiratory-chain complex 1, and via numerous downstream effects on mitochondrial function is thought to lead to support blood brain barrier integrity, enhance mechanisms of remyelination, inhibit neuronal apoptosis and possess anti-inflammatory properties [54,55]. Phase 1, 2a and phase 3 trials are now underway to investigate this further, including the recently opened OCTOPUS trial [56].
NICE guidance for diagnosing diabetes involves measuring HbA1c levels. For individuals without diabetes, NICE recommends HbA1c testing every 3-5 years, depending on age and risk factors. Screening for diabetes is also included in the NHS Health Check [57]. Diabetes is diagnosed at levels above 48 mmol/mol (6.5%) and the individual is considered at high risk of developing diabetes if the HbA1c level is 42-47 mmol/mol (6.0-6.4%) [58]. Diabetes management starts with education and comprehensive lifestyle modifications. Though this will of course be complemented by services provided by the GP, the routine MS clinic appointment again provides a useful opportunity to reiterate this advice and focus on the impact good diabetic control will have on their MS outcomes. We know that historically, among those with type 2 diabetes, PwMS had a 56% lower prevalence of antidiabetic usage [8]. Pharmacological treatment will typically be beyond the remit of neurology, but typically involves metformin, with additional oral or injectable medications added as needed to achieve optimal glycaemic control, such as sulfonylureas, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium-glucose co-transporter-2 (SGLT2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, or insulin (Figure 1) [58].
Osteoporosis
Bone metabolism disorders are probably under-recognised and under-treated in MS. Research shows a higher prevalence of osteoporosis and osteopenia in PwMS compared to those without the condition, and that these changes occur at a younger age [59,60]. People with progressive forms of MS appear to have more severe osteoporosis than those with relapsing–remitting MS (RRMS) [61].
Potential causes for the increased prevalence of osteoporosis in MS include shared risk factors (female gender, white race, Vitamin D deficiency), direct effects of MS (chronic inflammation, inactivity, recurrent falls and fractures, cognitive impairment, low body weight, visual impairment), and iatrogenic causes (including but not limited to glucocorticoid use) [62]. Low bone mass has however also been shown to occur early in MS (and even in clinically isolated syndrome) as well as in fully ambulant patients, suggesting also that there are possibly shared aetiological and pathogenic factors between the two conditions [63,64].
Osteoporosis is a major cause of morbidity and mortality in MS. The presence of osteoporosis is a significant predictive variable that a fall will result in fracture, and PwMS are at higher risk of fracture than general population [65]. Fractures can have significant impacts on the mobility of PwMS, with secondary deconditioning and long-term hospitalisation. In addition, reduced bone mineral density is associated with increased cognitive impairment in PwMS, suggesting a possible link between MS-related inflammatory and neurodegenerative processes and bone homeostasis [66].
PwMS are routinely prescribed vitamin D due to the association of low vitamin D levels with increased future risk of developing MS and with increased disease activity. Though vitamin D is essential for bone health, vitamin D supplementation alone in MS is not sufficient to prevent bone loss in those who are not vitamin D deficient, and therefore more active approaches to optimising bone health are required [67].
NICE guidelines for diagnosing osteoporosis involve using dual-energy X-ray absorptiometry (DEXA) to measure bone mineral density (BMD). The results are reported as T and Z scores, with osteoporosis defined as a T score of -2.5 standard deviations or lower, and osteopenia defined as a T score between -1 and -2.5 [68]. In the UK, there are no specific guidelines for how often healthy adults should have DEXA scans. However, it is generally recommended that postmenopausal women have a DEXA scan at age 65, and that men over 50 with risk factors for osteoporosis also have a scan.
A neurologist can of course organise a DEXA scan but may feel uncomfortable about interpretation of results or when to repeat the scan without other specialty input. The NICE guidance on the management of MS makes no reference to osteoporosis and reciprocally, scoring systems for bone density such as fracture risk assessment tool (FRAX) do not take MS into account. Bisson et al. showed that the FRAX score underestimates fracture risk in PwMS. Calibration of FRAX and fracture risk improved if osteoporosis was designated as “secondary” to MS, though MS is not currently listed among the secondary osteoporosis conditions [69].
Hearn et al. proposed a screening and management algorithm for osteoporosis in MS [70]. They suggest that anyone with MS who is felt to be at risk from deficiency should have their calcium and vitamin D status checked and replaced. Regarding further investigation, they recommend routine DEXA scans for postmenopausal women and for those with an EDSS over 6.0. They further recommend a high index of suspicion even in those with an EDSS less than 6.0 if they suffer a fracture, receive a prolonged (>3 month) course of glucocorticoid therapy or if they are on antiepileptic medication [70]. They recommend reviewing this at 1-2 yearly intervals. Treatment is with Vitamin D and Calcium preparations, and bisphosphonates (alendronate or risedronate) directed by NICE guidelines (Figure 1), with re-evaluation of a need for continued treatment (with repeat FRAX and/or DEXA) at five years [68].
Conclusion
Managing modifiable comorbidities is an important part of MS care that presents both challenges and opportunities. Comorbidities complicate treatment and compound disability in MS, but they also represent promising targets of (reasonably simple) intervention that can improve long-term health and quality of life. Neurologists and the MS multi-disciplinary team should incorporate the identification and management of modifiable comorbidity into routine MS care, where it need not take significant time or resources away from scheduled consults. Patient education, counselling, and referrals for further care and pharmacological intervention where necessary should become routine practice.
Although there are currently no specific guidelines for how often to screen for comorbidities in PwMS, we recommend regular screening for the discussed modifiable comorbidities, with shorter intervals in cases of concern. Screening need not be formal or repetitive if occurring elsewhere in other healthcare settings but comorbidities and their impact on MS should now be on the radar of the practicing neurologist.
Further research is needed to develop appropriate and MS targeted clinical screening algorithms for all modifiable comorbidities to enable early targeted interventions. Additional studies are needed to refine our understanding of how comorbid conditions contribute to MS progression and reciprocally, how MS contributes to the development of comorbidity.
References
- Kuhlmann T, et al. Multiple sclerosis progression: time for a new mechanism-driven framework. Lancet Neurol. 2023;22(1):78-88. https://doi.org/10.1016/S1474-4422(22)00289-7
- Dai D, Sharma A, Phillips AL, Lobo C. Patterns of Comorbidity and Multimorbidity Among Patients With Multiple Sclerosis in a Large US Commercially Insured and Medicare Advantage Population. J Health Econ Outcomes Res. 2022;9(2). https://doi.org/10.36469/001c.38669
- Marrie RA, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: Overview. Multiple Sclerosis Journal. 2015;21(3):263-281. https://doi.org/10.1177/1352458514564491
- Marrie RA, et al. Recommendations for observational studies of comorbidity in multiple sclerosis. Neurology. 2016;86(15):1446-1453. https://doi.org/10.1212/WNL.0000000000002474
- Marck CH, Neate SL, Taylor KL, Weiland TJ, Jelinek GA. Prevalence of Comorbidities, Overweight and Obesity in an International Sample of People with Multiple Sclerosis and Associations with Modifiable Lifestyle Factors. PLoS One. 2016;11(2) p. e0148573. https://doi.org/10.1371/journal.pone.0148573
- Edwards NC, Munsell M, Menzin J, Phillips AL. Comorbidity in US patients with multiple sclerosis. Patient Relat Outcome Meas. 2018;9:97-102. https://doi.org/10.2147/PROM.S148387
- Rotstein D, et al. High prevalence of comorbidities at diagnosis in immigrants with multiple sclerosis. Multiple Sclerosis Journal.2021;27(12):1902-1913. https://doi.org/10.1177/13524585211031791
- Palladino R, Marrie RA, Majeed A, Chataway J. Management of vascular risk in people with multiple sclerosis at the time of diagnosis in England: A population-based study. Multiple Sclerosis Journal. 2023;29(6):671-679. https://doi.org/10.1177/13524585231164296
- Marrie RA. Comorbidity in multiple sclerosis: Past, present and future. Clinical and Investigative Medicine. 2019;42(1):E5-E12. https://doi.org/10.25011/cim.v42i1.32383
- Marrie RA, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. Comorbidity delays diagnosis and increases disability at diagnosis in MS. Neurology. 2009;72(2):117-124. https://doi.org/10.1212/01.wnl.0000333252.78173.5f
- Zhang T et al. Examining the effects of comorbidities on disease-modifying therapy use in multiple sclerosis. Neurology. 2016;86(14):1287-1295. https://doi.org/10.1212/WNL.0000000000002543
- Tettey P et al. Frequency of Comorbidities and Their Association with Clinical Disability and Relapse in Multiple Sclerosis. Neuroepidemiology. 2016;46(2):106-113. https://doi.org/10.1159/000442203
- Kowalec K et al. Comorbidity increases the risk of relapse in multiple sclerosis. Neurology. 2017;89(24):2455-2461. https://doi.org/10.1212/WNL.0000000000004716
- Marrie RA et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010;74(13):1041-1047. https://doi.org/10.1212/WNL.0b013e3181d6b125
- Maric G et al. Impact of comorbidities on the disability progression in multiple sclerosis. Acta Neurol Scand. 2022;145(1):24-29. https://doi.org/10.1111/ane.13516
- Berrigan LI, et al. Health-related quality of life in multiple sclerosis. Neurology. 2016;86(15):1417-1424. https://doi.org/10.1212/WNL.0000000000002564
- Diržiuvienė B, Mickevičienė D. Comorbidity in multiple sclerosis: Emphasis on patient-reported outcomes. Mult Scler Relat Disord. 2022;59. p. 103558, https://doi.org/10.1016/j.msard.2022.103558
- Marrie RA et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology. 2015;85(3):240-247. https://doi.org/10.1212/WNL.0000000000001718
- Arneth B. Multiple Sclerosis and Smoking. Am J Med. 2020;133(7):783-788. https://doi.org/10.1016/j.amjmed.2020.03.008
- Panda SP, Das RC, Srivastava K, Ratnam A, Sharma N. Psychiatric comorbidity in multiple sclerosis. Neurol Neurochir Pol. 2018;52(6):704-709. https://doi.org/10.1016/j.pjnns.2018.09.003
- Marrie R, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. Comorbidity, socioeconomic status and multiple sclerosis. Multiple Sclerosis Journal. 2008;14(8)1091-1098. https://doi.org/10.1177/1352458508092263
- Allen NB, Lichtman JH, Cohen HW, Fang J, Brass LM, Alderman MH. Vascular Disease among Hospitalized Multiple Sclerosis Patients. Neuroepidemiology. 2008;30(4)234-238. https://doi.org/10.1159/000128103
- Kang J-H, Chen Y-H, Lin H-C. Comorbidities amongst patients with multiple sclerosis:a population‐based controlled study. Eur J Neurol. 2010;17(9):1215-1219. https://doi.org/10.1111/j.1468-1331.2010.02971.x
- Buchanan RJ, et al. Demographic and Disease Characteristics of People With Multiple Sclerosis Living in Urban and Rural Areas. Int J MS Care. 2006;8(3):89-97. https://doi.org/10.7224/1537-2073-8.3.89
- Briggs FBS, Hill E, Abboud H. The prevalence of hypertension in multiple sclerosis based on 37 million electronic health records from the United States. Eur J Neurol. 2021;28(2):558-566. https://doi.org/10.1111/ene.14557
- Centers for Disease Control and Prevention (CDC). Prevalence of disabilities and associated health conditions–United States, 1991-1992. MMWR Morb Mortal Wkly Rep. 1994;43(40)730-1,737-9.
- Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. J Hypertens. 2008;26(8):1636-1641. https://doi.org/10.1097/HJH.0b013e3283018333
- Williams T et al. Cardiovascular risk factors in secondary progressive multiple sclerosis: A cross‐sectional analysis from the <scp>MS‐STAT2</scp> randomized controlled trial. Eur J Neurol. 2023;30(9):2769-2780. https://doi.org/10.1111/ene.15924
- Conway DS, Thompson NR, Cohen JA. Influence of hypertension, diabetes, hyperlipidemia, and obstructive lung disease on multiple sclerosis disease course. Multiple Sclerosis Journal. 2017;23(2):277-285. https://doi.org/10.1177/1352458516650512
- Kappus N, et al. Cardiovascular risk factors are associated with increased lesion burden and brain atrophy in multiple sclerosis. J Neurol Neurosurg Psychiatry. p.jnnp-2014-310051, Feb. 2015. https://doi.org/10.1136/jnnp-2014-310051
- National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. Clinical guideline [CG181]. Accessed: Aug. 10, 2023. [Online]. Available: https://www.nice.org.uk/guidance/ng136/resources/visual-summary-pdf-6899919517
- Overs S, Hughes CM, Haselkorn JK, Turner AP. Modifiable Comorbidities and Disability in Multiple Sclerosis. Curr Neurol Neurosci Rep. 2012;12(5):610-617. https://doi.org/10.1007/s11910-012-0293-4
- Filippou CD, et al. Dietary Approaches to Stop Hypertension (DASH) Diet and Blood Pressure Reduction in Adults with and without Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Advances in Nutrition. 2020;11(5):1150-1160. https://doi.org/10.1093/advances/nmaa041
- Katz Sand I, Levy S, Fitzgerald K, Sorets T, Sumowski JF. Mediterranean diet is linked to less objective disability in multiple sclerosis. Multiple Sclerosis Journal. 2023;29(2):248-260. https://doi.org/10.1177/13524585221127414
- Marrie RA, Kosowan L, Singer A. Management of diabetes and hypertension in people with multiple sclerosis. Mult Scler Relat Disord. 2020;40. p. 101987 https://doi.org/10.1016/j.msard.2020.101987
- Marrie RA, Cutter G, Tyry T. Substantial adverse association of visual and vascular comorbidities on visual disability in multiple sclerosis. Multiple Sclerosis Journal. 2011;17(12):1464-1471. https://doi.org/10.1177/1352458511414041
- Marrie RA, et al. Differing trends in the incidence of vascular comorbidity in MS and the general population. Neurol Clin Pract 2016;6(2):120-128. https://doi.org/10.1212/CPJ.0000000000000230
- Marrie RA, et al. Rising prevalence of vascular comorbidities in multiple sclerosis: validation of administrative definitions for diabetes, hypertension, and hyperlipidemia. Multiple Sclerosis Journal. 2012;18(9):1310-1319. https://doi.org/10.1177/1352458512437814
- Weinstock-Guttman B, et al. Serum lipid profiles are associated with disability and MRI outcomes in multiple sclerosis. J Neuroinflammation. 2011;8(1):127. https://doi.org/10.1186/1742-2094-8-127
- Andaloro A, et al. Is there a correlation between dyslipidemia and cognitive impairment in patients with multiple sclerosis? International Journal of Neuroscience. 2022;132(2):201-206. https://doi.org/10.1080/00207454.2020.1807980
- Fitzgerald KC, Damian A, Conway D, Mowry EM. Vascular comorbidity is associated with lower brain volumes and lower neuroperformance in a large multiple sclerosis cohort. Multiple Sclerosis Journal. 2021;27(12):1914-1923. https://doi.org/10.1177/1352458520984746
- Lorefice L, et al. Assessing the burden of vascular risk factors on brain atrophy in multiple sclerosis: A case- control MRI study. Mult Scler Relat Disord. 2019;27:74-78. https://doi.org/10.1016/j.msard.2018.10.011
- Kowalec K et al. Comorbidity increases the risk of relapse in multiple sclerosis. Neurology. 2017;89(24):2455-2461. https://doi.org/10.1212/WNL.0000000000004716
- Salter A, Kowalec K, Fitzgerald KC, Cutter G, Marrie RA. Comorbidity is associated with disease activity in MS. Neurology. 2020;95(5)e446-e456. https://doi.org/10.1212/WNL.0000000000010024
- Sitia S, et al. From endothelial dysfunction to atherosclerosis. Autoimmun Rev. 2010;9(12):830-834. https://doi.org/10.1016/j.autrev.2010.07.016
- Williams T, et al. 007 The MS-STAT2 trial in secondary progressive MS – study design and update. J Neurol Neurosurg Psychiatry. 2022;93(6):A16.1-A16. https://doi.org/10.1136/jnnp-2022-ABN.46
- van der Most PJ, Dolga AM, Nijholt IM, Luiten PGM, Eisel UL. Statins: Mechanisms of neuroprotection. Prog Neurobiol. 2009;88(1):64-75. https://doi.org/10.1016/j.pneurobio.2009.02.002
- Chataway J et al. Efficacy of three neuroprotective drugs in secondary progressive multiple sclerosis (MS-SMART): a phase 2b, multiarm, double-blind, randomised placebo-controlled trial. Lancet Neurol. 2020;19(3):214-225. https://doi.org/10.1016/S1474-4422(19)30485-5
- National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Clinical guideline [CG181]. Accessed: Aug. 10, 2023. [Online]. Available: https://www.nice.org.uk/guidance/cg181
- Cardiovascular disease: risk assessment and reduction, including lipid modification. Jul. 2014.
- Hou W-H, Li C-Y, Chang H-H, Sun Y, Tsai C-C. A population-based cohort study suggests an increased risk of multiple sclerosis incidence in patients with type 2 diabetes mellitus. J Epidemiol. 2017;27(5):235-241. https://doi.org/10.1016/j.je.2016.06.006
- Wong E et al. Diabetes and risk of physical disability in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2013;1(2):106-114. https://doi.org/10.1016/S2213-8587(13)70046-9
- Marrie RA, et al. Diabetes and anxiety adversely affect cognition in multiple sclerosis. Mult Scler Relat Disord. 2019;27:164-170. https://doi.org/10.1016/j.msard.2018.10.018
- Negrotto L, Farez MF, Correale J. Immunologic Effects of Metformin and Pioglitazone Treatment on Metabolic Syndrome and Multiple Sclerosis. JAMA Neurol. 2016;73(5):520 https://doi.org/10.1001/jamaneurol.2015.4807
- Neumann B, et al. Metformin Restores CNS Remyelination Capacity by Rejuvenating Aged Stem Cells. Cell Stem Cell. 2019;25(4):473-485.e8. https://doi.org/10.1016/j.stem.2019.08.015
- “OCTOPUS Trial website,” MRCCTU. Accessed: Jun. 14, 2023. [Online]. Available: https://ms-octopus.mrcctu.ucl.ac.uk/
- NHS Health Check, “The NHS Health Check.” Accessed: Oct. 20, 2023. [Online]. Available: https://www.healthcheck.nhs.uk
- National Institute for Health and Care Excellence, “Type 2 diabetes in adults: management,” NICE guideline [NG28]. Accessed: Aug. 10, 2023. [Online]. Available: https://www.nice.org.uk/guidance/ng28
- Bisson EJ, Finlayson ML, Ekuma O, Leslie WD, Marrie RA. Multiple sclerosis is associated with low bone mineral density and osteoporosis. Neurol Clin Pract. 2019;9(5):391-399. https://doi.org/10.1212/CPJ.0000000000000669
- Murphy O, Zandi MS, Lindenberg N, Murphy E, Chataway J. Bone health in patients with multiple sclerosis relapses. Mult Scler Relat Disord. 2016;6:75-80 .https://doi.org/10.1016/j.msard.2016.02.003
- Nieves J, Cosman F, Herbert J, Shen V, Lindsay R. High prevalence of vitamin D deficiency and reduced bone mass in multiple sclerosis. Neurology. 1994;44(9):1687-1687. https://doi.org/10.1212/WNL.44.9.1687
- Gupta S, Ahsan I, Mahfooz N, Abdelhamid N, Ramanathan M, Weinstock-Guttman B. Osteoporosis and Multiple Sclerosis: Risk Factors, Pathophysiology, and Therapeutic Interventions. CNS Drugs. 2014;28(8):731-742. https://doi.org/10.1007/s40263-014-0173-3
- Sioka C, et al. Bone mineral density in ambulatory patients with multiple sclerosis. Neurological Sciences. 2011;32(5):819-824. https://doi.org/10.1007/s10072-011-0623-3
- Moen SM, Celius EG, Sandvik L, Nordsletten L, Eriksen EF, Holmoy T. Low bone mass in newly diagnosed multiple sclerosis and clinically isolated syndrome. Neurology. 2011;77(2):151-157. https://doi.org/10.1212/WNL.0b013e3182242d34
- Cosman F, et al. Fracture history and bone loss in patients with MS. Neurology. 1998;51(4):1161-1165. https://doi.org/10.1212/WNL.51.4.1161
- Batista B, et al. Cognitive impairment is associated with reduced bone mass in multiple sclerosis. Multiple Sclerosis Journal. 2012;18(10):1459-1465. https://doi.org/10.1177/1352458512440206
- Holmøy T, Lindstrøm JC, Eriksen EF, Steffensen LH, Kampman MT. High dose vitamin D supplementation does not affect biochemical bone markers in multiple sclerosis – a randomized controlled trial. BMC Neurol. 2017;17(1):67. https://doi.org/10.1186/s12883-017-0851-0
- National Institute for Health and Care Excellence. Osteoporosis – prevention of fragility fractures. Clinical Knowledge Summaries. Accessed: Aug. 10, 2023. [Online]. Available: https://cks.nice.org.uk/topics/osteoporosis-prevention-of-fragility-fractures/
- Bisson EJ, Finlayson ML, Ekuma O, Marrie RA, Leslie WD. Accuracy of FRAX ® in People With Multiple Sclerosis. Journal of Bone and Mineral Research. 2019;34(6):1095-1100. https://doi.org/10.1002/jbmr.3682
- Hearn AP, Silber E. Osteoporosis in multiple sclerosis. Multiple Sclerosis Journal. 2010;16(9):1031-1043. https://doi.org/10.1177/1352458510368985



