Abstract
Gait disorders are a frequent feature of neurology clinics, and are becoming more prominent within an ageing population. Gait is controlled by deep, evolutionarily ancient systems working in unison, predicting and enacting a walking model. Naturalistic gait involves multi-tasking and responding to environmental challenges, requiring higher cognitive processing. The control of gait is highly interconnected and so gait disorders may result from a wide array of neurological insults. This review provides a succinct summary of the underlying neurophysiology of gait for the busy clinician. We explore the neural networks controlling walking, from automated spinal cord networks through to cortical planning. Throughout, we highlight clinical phenotypes resulting from injury at each anatomical level and discuss future directions for the field.
Introduction
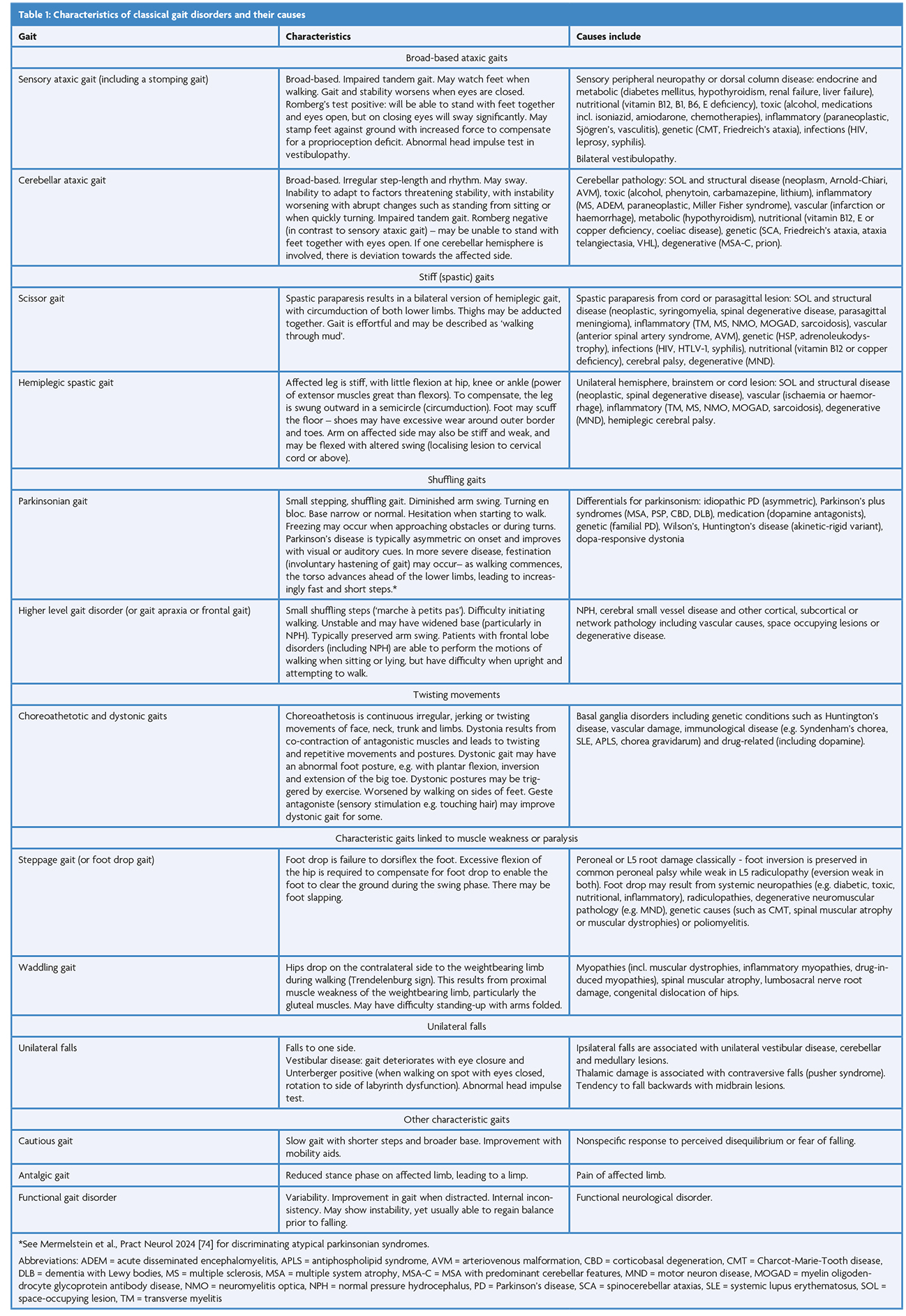
Gait disorders carry significant functional ramifications and are very common, affecting ~35% of those aged 70 and older [1] and ~60% of neurology inpatients [2]. The examination of gait is a critical part of the neurological examination (Table 1) [3–6], informing diagnosis and rehabilitation. Gait characteristics may also have a role in prognostication, such as assessing dementia risk [7]. In this context, it is increasingly important for clinicians to understand the neural control for gait and how pathology may result. We will tackle the network anatomically, and start close to our effector muscles, deep within the spinal cord (Figure 1).
Spinal central pattern generators activate muscles
Walking requires coordination of many muscle groups across multiple joints. This can be orchestrated by the spinal cord as was shown by the seminal work of Graham Brown in 1911, where decerebrated cat preparations had the same phases of movement with and without their dorsal roots severed [8]. This demonstrated that there are networks within the spinal cord capable of generating walking movements. These networks are central pattern generators (CPGs), and are composed of rhythmically firing interneurons. There are flexor-extensor CPGs for intralimb coordination, and left-right CPGs to coordinate the legs [9]. Initially CPGs were conceptualised as reciprocally inhibiting groupings (or “half-centres”) of interneurons, but these concepts have evolved to consider separate rhythm- and pattern-generating circuitry [10,11]. A recent phase I/IIa clinical trial for patients with motor complete spinal cord injury used Spinalon™ (buspirone/levodopa/carbidopa) to target CPGs. Excitingly, some patients demonstrated rhythmic flexor-extensor activity, supporting a role for CPGs in humans [12]. Proprioceptive and cutaneous sensory afferents feed into spinal networks, including CPG interneurons [13]. They influence the timing and amplitude of locomotive activity, and are important for regulating the stance and swing phases [13]. Impairment of sensory pathways can lead to a sensory ataxic gait, as described in Table 1. Damage to the spinal cord itself may instead cause a spastic gait. Spasticity has multiple underlying mechanisms, but loss of reticulospinal inhibition to stretch reflex arcs is a significant factor [14]. In summary, CPGs are spinal interneuron networks that coordinate intralimb and interlimb movements and, in some mammals, stimulation can drive walking.
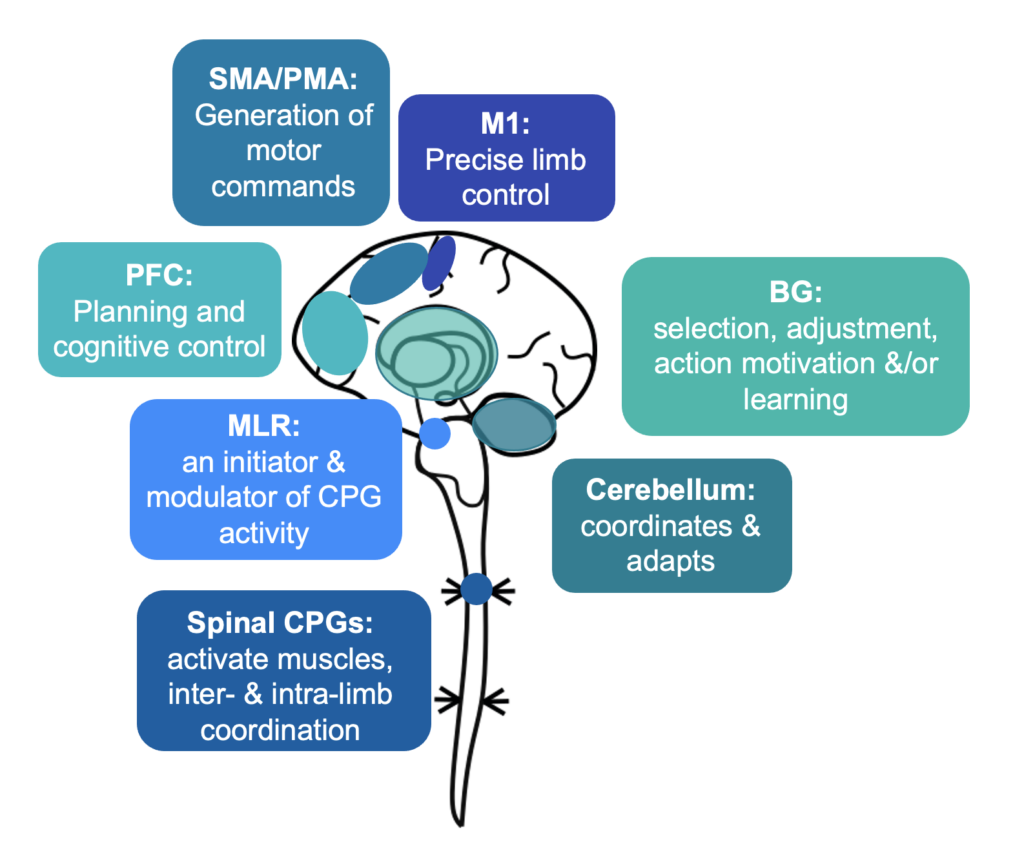
Figure 1: The roles of neural networks involved in gait – Spinal CPGs activate muscles via motor neurons, and coordinate multiple muscle groups to walk. The MLR is an initiator of gait and a controller in the process of walking. The cerebellum supports multi-joint functionality and can adapt and respond to external challenges to gait as they develop. The basal ganglia may regulate and adjust motor programmes, deliver the motivation or vigour for movement, and support procedural learning. The cortex integrates internal and external motivators to walk, with the premotor area generating motor commands and the prefrontal cortex involved in planning and cognitive control. The primary motor cortex delivers precise limb movements. CPG = central pattern generator; MLR = mesencephalic locomotor region, PFC = prefrontal cortex, SMA/PMA = premotor area including supplementary motor area, M1 = primary motor cortex, BG = basal ganglia; Figure incorporates 10.5281/zenodo.4724290 from Jon Perdomo on Scidraw.io.
The mesencephalic locomotor region – control of spinal central pattern generators
The automatic processes of CPGs require supraspinal modulation. An area within the midbrain and upper brainstem named the mesencephalic locomotor region (MLR) was proposed as a key initiator for gait, following electrical stimulation experiments eliciting controlled walking and running in a cat [15]. The MLR involves neurons within the pedunculopontine (PPN) and cuneiform nuclei (CnF), with input from the basal ganglia, amygdala, bed nucleus of the stria terminalis and lateral hypothalamus [16]. The functions of the MLR range from postural tone to controlling the initiation, rhythm and speed of gait [17,18]. Overall, the CnF is associated with fast movements and the PPN with slower movements (Figure 2), though analysis of cellular subpopulations adds greater detail. Glutamatergic neurons in the CnF are associated with initiating gait and promoting faster walking while glutamatergic (and to some extent cholinergic) PPN neurons may promote slower walking [19,20] or support the stance phase [21,22]. An MLR GABAergic neuronal population inhibits locomotion [23]. The outputs from the PPN and CnF may also differ, with the CnF having a more localised output while the PPN may form more widespread networks, including with the basal ganglia and spinal networks via the reticulospinal tract [22]. Overall, the MLR and brainstem nuclei adjust CPG activity via the reticulospinal tract, vestibulospinal tract, tectospinal tract and monoaminergic pathways [16]. The PPN has become an experimental target for deep brain stimulation (DBS), with results highly variable between patients, although showing potential improvements for gait freezing and falls for some [24]. The MLR together has a role in initiating locomotion, with its constituent nuclei potentially favouring escape (CnF) and exploratory (PPN) behaviour [21].
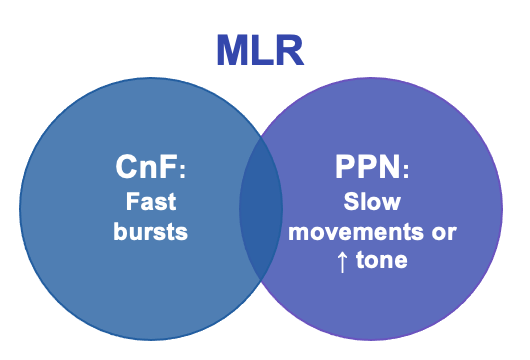
Figure 2: Diagram illustrating how MLR consists of CN and PPN with closely intertwined functionality. MLR = mesencephalic locomotor region, CN = cuneiform nucleus, PPN = pedunculopontine nucleus
Cerebellum coordinates walking and responds to challenges
Cerebellar lesions cause an ataxic gait, characterised by disordered multi-joint coordination. This highlights the role of the cerebellum in the control of limb movements and balance [25]. Lesions affect multi-joint functionality rather than specific muscles, or in other words cerebellar regions control actions rather than muscles in anatomical proximity [26,27]. The cerebellum projects largely to the brainstem, thalamus and spinal cord; direct projections which may be of importance for gait include from the fastigial nucleus to the vestibular nuclei and spinal cord, and from the dentate nucleus to the reticular nuclei [26].
The role of the cerebellum in coordination relies on its ability to learn motor sequences, preventing the need to consciously decompose every action. This learning occurs at Purkinje cells, which integrate information from two key cell types: climbing fibres (from the inferior olive) and granule cells with their parallel fibres (receiving input from mossy fibres) (Figure 3). Parallel fibres relay an efference copy of motor commands and provide sensory context. A single climbing fibre wraps itself like ivy around a Purkinje cell, provides error feedback (a teaching signal), and its firing triggers a Purkinje cell action potential [28,29]. Learning occurs through climbing fibre activation depressing simultaneous parallel fibre inputs, termed long-term depression (LTD) [30,31]. Disruption of LTD has been shown to specifically impact the adaptability of gait [32]. The cerebellum is important for responding to unexpected challenges to gait in humans: cerebellar patients respond irregularly to alterations in treadmill speed, while controls respond in rhythm with the normal locomotor cycle [33].
The cerebellum may also influence the initiation of gait. Stimulation of a restricted region of midline cerebellar white matter (the hook bundle of Russell) produced well-coordinated, bilaterally symmetrical, fore- and hind-limb movements in a supported decerebrated cat. This was evident even with MLR ablation [34], indicating that this ‘cerebellar locomotor region (CLR)’ may act through an independent pathway to the MLR. Overall, the cerebellum acts to coordinate multi-joint movements and respond to postural challenges.
Figure 3: Diagrammatic illustration of cerebellar cortex cells
Basal ganglia may select motor commands, adjust movements, deliver action motivation, and/or contribute to motor learning
The basal ganglia (BG) are central to the understanding of movement disorders: substantia nigra atrophy and dopamine loss in Parkinson’s disease is associated with a paucity of movement (including shuffling gait), while striatal degeneration is associated with hyperkinesis in Huntington’s disease [35]. The BG do not initiate movement, as the output region of the BG, the internal globus pallidus, is active after the onset of muscle contraction. The anatomical circuitry of the BG has favoured a ‘brake-accelerator model’: an indirect pathway (striatum D2 to external pallidum to subthalamic nucleus to internal pallidum) inhibits the thalamus, while a direct pathway (striatum D1 to internal pallidum) releases this inhibition (Figure 4). Running with this model, the BG may disinhibit the desired movement while inhibiting undesired movements [36]. One supportive example is how GABAergic fast-spiking interneurons (FSIs) in the striatum fire when a chosen action is initiated and a highly trained alternative is suppressed [37]. The BG project to the MLR and can activate or suppress MLR glutamatergic neurons, as would be required for such a model [23]. However, some have argued that the BG may not be active early enough in movement planning for this role [38]. The BG have also been proposed to adjust the speed and size of movement, which may account for the signs of bradykinesia, micrographia and hypophonia in Parkinson’s disease [39]. Alternatively, the BG may be involved in movement cost-reward calculations, and so influence motivation or vigour [38]. This may explain how people with Parkinson’s disease may be capable of moving as quickly as healthy individuals, but are naturally bradykinetic [38,40]. The BG further act in procedural learning (rather than retention or recall) [38], with long term potentiation and depression occurring in the striatum [41]. Altogether, the roles of the BG may include selecting desired motor commands, adjusting the speed and size of movements, delivering the motivation or vigour for movement, and/or motor learning.
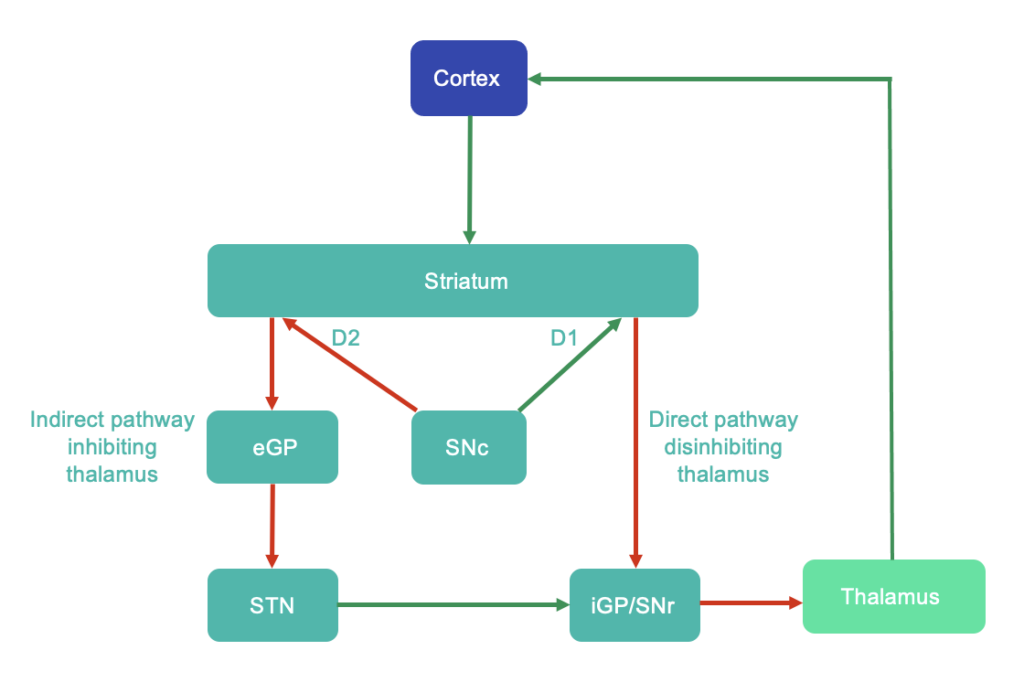
Figure 4: Traditional concept of basal ganglia circuitry – direct and indirect pathways. Green arrows indicate excitatory glutamatergic pathways, while red arrows indicate GABAergic inhibitory pathways. D1 and D2 indicate dopamine receptor subtypes. This is known to be a simplification, with further modulatory transverse pathways present, e.g. see Lanciego et al., 2012 [75]. Abbreviations: eGP = external globus pallidus, iGP = internal globus pallidus, SNc = substantia nigra pars compacta, SNr = substantia nigra pars reticulata, STN = subthalamic nucleus
Cortex – deciding to walk
The cortex acts in the preparation, decision and initiation phases of gait. It weighs up the motivational drive to walk with the social and environmental context. The prefrontal cortex (PFC) is a key region for this goal-directed executive decision making [16,42]. The supplementary motor areas (SMA) and other premotor area (PMA) regions generate the motor commands following communication from the PFC. This is conveyed by corticoreticular fibres to the MLR and brainstem reticular formation, and in turn to CPGs. In parallel, the SMA/PMA communicates with M1, which controls foot and precise limb movements via the corticospinal tract [16,17] (Figure 1). This spreading recruitment of cortical regions can be seen on electroencephalography (EEG) and is termed the Bereitschaftspotential or readiness potential (RP), and is seen approximately two seconds prior to movement [43,44]. Of note, sensory information about the external environment, and the location of the body within it, requires input from all sensory modalities. Significant sensory processing occurs in the parietal cortex [16,45]. In keeping with the role of the cortex in the initiation of gait, higher level gait disorders (HLGD) have a phenotype including hesitant starts and turns [6,46].
M1 is active during the conscious drive to move, particularly for fine motor tasks. It has a somatotopic architecture (homunculus), with specific M1 regions linked with movements of distinct regions of the body [47]. Giant pyramidal neurons characterise M1, and these fast acting neurons synapse directly on anterior horn motor neurons or on associated spinal interneurons, enabling rapid and specific movements [48].The corticospinal tract is not however composed solely of M1 axons, but includes axons from the SMA, superior parietal lobule and primary somatosensory cortex [4]. Similarly not all M1 pyramidal neurons project to the corticospinal tract: some have projections across the cortex, basal ganglia, cerebellum and brainstem [49], some projecting to multiple distant sites [50]. Additionally, there may be highly connected control areas interspersed between motor control regions in M1. This new model (proposed by Gordon et al., Nature 2023) offers a tantalising means by which motor commands may be integrated with whole-body, metabolic and physiological control [51].
The SMA/PMA are important for motor programming and generating motor commands. The premotor area has been associated with sequencing tasks and reward-directed movements (specifically pre-SMA and dorsal premotor areas) [52]. These regions are important for switching away from routine movements when the environment changes and that routine is no longer appropriate [53]. The SMA helps prepare for the centre of gravity moving during walking (anticipatory postural adjustment) [16]. The caudal premotor area including the SMA maintains a somatotopic representation, although not as clear as that of M1.
The PFC has widespread functions in gait as an executive region, with roles in decision making, attention, working memory, planning task sequencing and personality [16,42]. Increased PFC activation has been consistently noted in dual task walking, reflecting its role in attention. Unlike walking on a treadmill in a controlled setting, navigating the real world requires a constant interplay between planning and execution: we avoid static and moving obstacles, often while talking. The gait pattern of healthy young adults changes when dual tasking; this would only be expected if higher cognitive attention is required for walking [54]. For those with stroke, multiple sclerosis, or in healthy older adults, even normal walking has been associated with increased PFC activation, which may represent a compensatory mechanism [49,50]. The PFC is integrated into the limbic or emotional network through connections with the hypothalamus and periaqueductal grey, critical for goal-directed naturalistic walking [16]. Further centres included in this network are the amygdala, hippocampus and nucleus accumbens, incorporating emotional drivers for gait.
An exceptional recent review (Gait control by the frontal lobe, Handbook of Clinical Neurology, Takakusaki) highlighted how the prefrontal and premotor areas have extensive connectivity across the central nervous system. Key pathways include a parieto-prefrontal (‘where’) pathway transferring spatial information to the PFC and a parieto-premotor (‘how’) pathway for visually guided movements. Further cortical networks important for gait include an occipito-temporal (‘what’) pathway for visual processing and a parieto-medial temporal pathway for route navigation and long-term spatial memory [16].
In summary, as walking is a purposeful action within the environment, it involves not only motor cortex activity, but also sensory systems and higher cognitive processing. It is therefore unsurprising that neurological disorders so commonly cause gait disturbance.

Figure 5: an example of a wearable OPM-MEG being used
to investigate cortical activity at the Sir Peter Mansfield Imaging Centre, University of Nottingham
Neuroimaging approaches are furthering our understanding of gait disorders
Our understanding of gait disorders is still limited by our inability to image individuals while moving with high temporal resolution. This is especially evident for HLGD, where our knowledge is particularly limited [55].
One approach has been to utilise EEG to investigate cortical activity in gait. Through this, researchers have characterised the electrical activity when standing [56], during the gait cycle [57] and even with distractions when walking across a university campus [58]. EEG has further been thoroughly utilised to investigate disease states, such as freezing of gait in Parkinson’s disease [59]. The utility of EEG however is limited by noise and its poorer spatial resolution when compared with other modalities.
Functional near-infrared spectroscopy (fNIRS) has been extensively used for investigating the neuroscience of movement [60]. Near-infrared light determines haemoglobin concentrations in tissues, and so their aerobic metabolic demand. There may be a time-lag of 4-7 seconds between cortical activity and haemodynamic response however [61–63]. fNIRS can offer improved spatial resolution compared to EEG (previously estimated as 5mm vs 10mm) [64]. Similar to EEG, fNIRS is appropriate for studies of the cortex rather than deeper structures, as it relies on the penetration of infrared light to those tissues [64]. fNIRS has been applied for researching gait [65], preparation for walking [66], gait in disease states [67] and dual task walking [68].
Another approach taken has been to utilise [18F]-fluorodeoxyglucose (FDG) positron emission tomography (PET), whereby subjects walk, are injected with tracer, then continue walking, following which imaging is performed. This relies on cerebral glucose utilisation being weighted to the first 10-15 minutes after [18F]-FDG injection [69]. The Newcastle group using PET [70] have reported two resting covariance networks associated with gait characteristics in Parkinson’s disease [71].
Magnetoencephalography with optically pumped magnetometers (OPM-MEG) offers a novel approach to investigate movement and could prove a real game-changer in the study of gait. It offers a finer spatial resolution than EEG and greater temporal resolution than fNIRS: MEG has quoted temporal resolution in the millisecond range and spatial resolution of c.2–5 mm [72]. The spatial resolution is not as detailed as fMRI for deep structures, although new approaches are ongoing for enhancing this, with a recent study adapting OPM-MEG to analyse the hippocampus [73]. OPM-MEG is integrated into a wearable helmet, offering an exciting opportunity to investigate gait disorders directly (as shown in Figure 4) – only time will tell if it will realise a role as a functional neuroimaging tool for gait.
Conclusions
Locomotion relies on deep and interconnected neural networks. The MLR initiates gait through spinal CPGs. This is informed by cortical regions, particularly prefrontal and premotor areas, integrating sensory information with the desire and motivation to walk. The cerebellum coordinates walking and adapts to challenges. The basal ganglia may have roles in selecting motor commands, adjusting movements, delivering action motivation, and/or contributing to motor learning, Together, these different regions work in unison, predicting and enacting a walking model. Naturalistic gait is goal-directed and responsive to a changing environment, requiring higher cognitive processing. Given how interdependent all these regions are for walking, gait disorders result from disruption of any part of this pathway from cortex to muscle. OPM-MEG offers the potential opportunity to measure gait disorders with greater spatial resolution than EEG and finer temporal resolution than fNIRS.
References
- Verghese J, LeValley A, Hall CB, Katz MJ, Ambrose AF, Lipton RB. Epidemiology of Gait Disorders in Community-Residing Older Adults. J Am Geriatr Soc. 2006;54(2):255-261. https://doi.org/10.1111/j.1532-5415.2005.00580.x
- Stolze H, Klebe S, Baecker C, et al. Prevalence of gait disorders in hospitalized neurological patients. Mov Disord. 2005;20(1):89-94. https://doi.org/10.1002/mds.20266
- Pirker W, Katzenschlager R. Gait disorders in adults and the elderly. Wien Klin Wochenschr. 2017;129(3):81-95. https://doi.org/10.1007/s00508-016-1096-4
- Allan H. Ropper, Martin A. Samuels. Adams and Victor’s Principles of Neurology. 9th ed. McGraw-Hill Education; 2009.
- Neurological gait disorders in elderly people: clinical approach and classification. Lancet Neurol. 2007;6(1):63-74. https://doi.org/10.1016/S1474-4422(06)70678-0
- Baker JM. Gait Disorders. Am J Med. 2018;131(6):602-607. https://doi.org/10.1016/j.amjmed.2017.11.051
- Gait analysis as a clinical tool for dementia: current perspectives and future challenges. ACNR Adv Clin Neurosci Rehabil. Published online May 21, 2021. Accessed July 20, 2023. https://acnr.co.uk/articles/gait-analysis/ https://doi.org/10.47795/OBXA9271
- Brown TG. The intrinsic factors in the act of progression in the mammal. Proc R Soc Lond Ser B Contain Pap Biol Character. 1911;84(572):308-319. https://doi.org/10.1098/rspb.1911.0077
- Minassian K, Hofstoetter US, Dzeladini F, Guertin PA, Ijspeert A. The Human Central Pattern Generator for Locomotion: Does It Exist and Contribute to Walking? The Neuroscientist. 2017;23(6):649-663. https://doi.org/10.1177/1073858417699790
- Grillner S, Wallén P. Central pattern generators for locomotion, with special reference to vertebrates. Annu Rev Neurosci. 1985;8. https://doi.org/10.1146/annurev.ne.08.030185.001313
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev. 2008;57(1):134-146. https://doi.org/10.1016/j.brainresrev.2007.08.006
- Radhakrishna M, Steuer I, Prince F, et al. Double-Blind, Placebo-Controlled, Randomized Phase I/IIa Study (Safety and Efficacy) with Buspirone/Levodopa/Carbidopa (SpinalonTM) in Subjects with Complete AIS A or Motor-Complete AIS B Spinal Cord Injury. Curr Pharm Des. 2017;23(12):1789-1804. https://doi.org/10.2174/1381612822666161227152200
- Frigon A, Rossignol S. Experiments and models of sensorimotor interactions during locomotion. Biol Cybern. 2006;95(6):607-627. https://doi.org/10.1007/s00422-006-0129-x
- Chakravarty A, Mukherjee A. Spasticity Mechanisms – for the Clinician. Front Neurol. 2010;1. Accessed July 17, 2023. https://www.frontiersin.org/articles/10.3389/fneur.2010.00149 https://doi.org/10.3389/fneur.2010.00149
- Shik ML, Severin FV, Orlovskiĭ GN. [Control of walking and running by means of electric stimulation of the midbrain]. Biofizika. 1966;11(4):659-666.
- Takakusaki K. Gait control by the frontal lobe. Handb Clin Neurol. 2023;195:103-126. https://doi.org/10.1016/B978-0-323-98818-6.00021-2
- Takakusaki K. Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov Disord Off J Mov Disord Soc. 2013;28(11):1483-1491. https://doi.org/10.1002/mds.25669
- Lau B, François C, Karachi C. Structure and function of the mesencephalic locomotor region in normal and parkinsonian primates. Curr Opin Physiol. 2019;8:121-128. https://doi.org/10.1016/j.cophys.2019.01.010
- Caggiano V, Leiras R, Goñi-Erro H, et al. Midbrain circuits that set locomotor speed and gait selection. Nature. 2018;553(7689):455-460. https://doi.org/10.1038/nature25448
- Josset N, Roussel M, Lemieux M, Lafrance-Zoubga D, Rastqar A, Bretzner F. Distinct Contributions of Mesencephalic Locomotor Region Nuclei to Locomotor Control in the Freely Behaving Mouse. Curr Biol. 2018;28(6):884-901.e3. https://doi.org/10.1016/j.cub.2018.02.007
- Ali F, Benarroch E. What Is the Brainstem Control of Locomotion? Neurology. 2022;98(11):446-451. https://doi.org/10.1212/WNL.0000000000200108
- Dautan D, Kovács A, Bayasgalan T, Diaz-Acevedo MA, Pal B, Mena-Segovia J. Modulation of motor behavior by the mesencephalic locomotor region. Cell Rep. 2021;36(8):109594. https://doi.org/10.1016/j.celrep.2021.109594
- Roseberry TK, Lee AM, Lalive AL, Wilbrecht L, Bonci A, Kreitzer AC. Cell-Type-Specific Control of Brainstem Locomotor Circuits by Basal Ganglia. Cell. 2016;164(3):526-537. https://doi.org/10.1016/j.cell.2015.12.037
- Thevathasan W, Debu B, Aziz T, et al. Pedunculopontine nucleus deep brain stimulation in Parkinson’s disease: A clinical review. Mov Disord. 2018;33(1):10-20. https://doi.org/10.1002/mds.27098
- Ilg W, Giese MA, Gizewski ER, Schoch B, Timmann D. The influence of focal cerebellar lesions on the control and adaptation of gait. Brain J Neurol. 2008;131(Pt 11):2913-2927. https://doi.org/10.1093/brain/awn246
- Thomas Thach W, Bastian AJ. Role of the cerebellum in the control and adaptation of gait in health and disease. In: Progress in Brain Research. Vol 143. Brain Mechanisms for the Integration of Posture and Movement. Elsevier; 2004:353-366. https://doi.org/10.1016/S0079-6123(03)43034-3
- Botterell EH, Fulton JF. Functional localization in the cerebellum of primates III. Lesions of hemispheres (neocerebellum). J Comp Neurol. 1938;69(1):63-87. https://doi.org/10.1002/cne.900690106
- Dean P, Porrill J. Adaptive-filter Models of the Cerebellum: Computational Analysis. The Cerebellum. 2008;7(4):567-571. https://doi.org/10.1007/s12311-008-0067-3
- Surdin T, Preissing B, Rohr L, et al. Optogenetic activation of mGluR1 signaling in the cerebellum induces synaptic plasticity. iScience. 2023;26(1):105828. https://doi.org/10.1016/j.isci.2022.105828
- Ito M. Long-term depression. Annu Rev Neurosci. 1989;12:85-102. https://doi.org/10.1146/annurev.ne.12.030189.000505
- Yanagihara D, Kondo I. Nitric oxide plays a key role in adaptive control of locomotion in cat. Proc Natl Acad Sci U S A. 1996;93(23):13292-13297. https://doi.org/10.1073/pnas.93.23.13292
- Rand MK, Wunderlich DA, Martin PE, Stelmach GE, Bloedel JR. Adaptive changes in responses to repeated locomotor perturbations in cerebellar patients. Exp Brain Res. 1998;122(1):31-43. https://doi.org/10.1007/s002210050488
- Mori S, Matsui T, Kuze B, Asanome M, Nakajima K, Matsuyama K. Stimulation of a restricted region in the midline cerebellar white matter evokes coordinated quadrupedal locomotion in the decerebrate cat. J Neurophysiol. 1999;82(1):290-300. https://doi.org/10.1152/jn.1999.82.1.290
- Papoutsi M, Labuschagne I, Tabrizi SJ, Stout JC. The cognitive burden in Huntington’s disease: Pathology, phenotype, and mechanisms of compensation. Mov Disord. 2014;29(5):673-683. https://doi.org/10.1002/mds.25864
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50(4):381-425. https://doi.org/10.1016/S0301-0082(96)00042-1
- Gage GJ, Stoetzner CR, Wiltschko AB, Berke JD. Selective activation of striatal fast-spiking interneurons during choice execution. Neuron. 2010;67(3):466-479. https://doi.org/10.1016/j.neuron.2010.06.034
- Turner RS, Desmurget M. Basal Ganglia Contributions to Motor Control: A Vigorous Tutor. Curr Opin Neurobiol. 2010;20(6):704-716. https://doi.org/10.1016/j.conb.2010.08.022
- Spraker MB, Yu H, Corcos DM, Vaillancourt DE. Role of individual basal ganglia nuclei in force amplitude generation. J Neurophysiol. 2007;98(2):821-834. https://doi.org/10.1152/jn.00239.2007
- Mazzoni P, Hristova A, Krakauer JW. Why don’t we move faster? Parkinson’s disease, movement vigor, and implicit motivation. J Neurosci Off J Soc Neurosci. 2007;27(27):7105-7116. https://doi.org/10.1523/JNEUROSCI.0264-07.2007
- Lerner TN, Kreitzer AC. Neuromodulatory control of striatal plasticity and behavior. Curr Opin Neurobiol. 2011;21(2):322-327. https://doi.org/10.1016/j.conb.2011.01.005
- Carlén M. What constitutes the prefrontal cortex? Science. 2017;358(6362):478-482. https://doi.org/10.1126/science.aan8868
- Shibasaki H, Hallett M. What is the Bereitschaftspotential? Clin Neurophysiol. 2006;117(11):2341-2356. https://doi.org/10.1016/j.clinph.2006.04.025
- Armstrong S, Sale MV, Cunnington R. Neural Oscillations and the Initiation of Voluntary Movement. Front Psychol. 2018;9. Accessed April 14, 2023. https://www.frontiersin.org/articles/10.3389/fpsyg.2018.02509 https://doi.org/10.3389/fpsyg.2018.02509
- Drew T, Marigold DS. Taking the next step: cortical contributions to the control of locomotion. Curr Opin Neurobiol. 2015;33:25-33. https://doi.org/10.1016/j.conb.2015.01.011
- Nutt JG. Higher-level gait disorders: An open frontier. Mov Disord. 2013;28(11):1560-1565. https://doi.org/10.1002/mds.25673
- Penfield W, Boldrey E. Somatic Motor and Sensory Representation in the in the Cerebral Cortex of Man as studied by Electrical Stimulation. Brain. 1937;60(4):389-443. https://doi.org/10.1093/brain/60.4.389
- Squire L, Berg D, Bloom FE, et al. Fundamental Neuroscience. Academic Press; 2008.
- Park J, Phillips JW, Guo JZ, Martin KA, Hantman AW, Dudman JT. Motor cortical output for skilled forelimb movement is selectively distributed across projection neuron classes. Sci Adv. 2022;8(10):eabj5167. https://doi.org/10.1126/sciadv.abj5167
- Winnubst J, Bas E, Ferreira TA, et al. Reconstruction of 1,000 projection neurons reveals new cell types and organization of long-range connectivity in the mouse brain. Cell. 2019;179(1):268-281.e13. https://doi.org/10.1016/j.cell.2019.07.042
- Gordon EM, Chauvin RJ, Van AN, et al. A somato-cognitive action network alternates with effector regions in motor cortex. Nature. 2023;617(7960):351-359. https://doi.org/10.1038/s41586-023-05964-2
- Hikosaka O, Isoda M. Switching from automatic to controlled behavior: cortico-basal ganglia mechanisms. Trends Cogn Sci. 2010;14(4):154-161. https://doi.org/10.1016/j.tics.2010.01.006
- Nakajima T, Hosaka R, Mushiake H. Complementary Roles of Primate Dorsal Premotor and Pre-Supplementary Motor Areas to the Control of Motor Sequences. J Neurosci. 2022;42(36):6946-6965. https://doi.org/10.1523/JNEUROSCI.2356-21.2022
- Srygley JM, Mirelman A, Herman T, Giladi N, Hausdorff JM. When does walking alter thinking? Age and task associated findings. Brain Res. 2009;1253:92-99. https://doi.org/10.1016/j.brainres.2008.11.067
- Walusinski O. From hysteria to gait dementia: History of the concept of astasia-abasia. Rev Neurol (Paris). 2023;179(6):523-532. https://doi.org/10.1016/j.neurol.2022.12.012
- Zhavoronkova LA, Zharikova AV, Kushnir EM, Mikhalkova AA. EEG markers of upright posture in healthy individuals. Hum Physiol. 2012;38(6):604-612. https://doi.org/10.1134/S0362119712050131
- Gwin JT, Gramann K, Makeig S, Ferris DP. Electrocortical activity is coupled to gait cycle phase during treadmill walking. NeuroImage. 2011;54(2):1289-1296. https://doi.org/10.1016/j.neuroimage.2010.08.066
- Liebherr M, Corcoran AW, Alday PM, et a l. EEG and behavioral correlates of attentional processing while walking and navigating naturalistic environments. Sci Rep. 2021;11(1):22325. https://doi.org/10.1038/s41598-021-01772-8
- Stuart S, Wagner J, Makeig S, Mancini M. Brain Activity Response to Visual Cues for Gait Impairment in Parkinson’s Disease: An EEG Study. Neurorehabil Neural Repair. 2021;35(11):996-1009. https://doi.org/10.1177/15459683211041317
- Hamacher D, Herold F, Wiegel P, Hamacher D, Schega L. Brain activity during walking: A systematic review. Neurosci Biobehav Rev. 2015;57:310-327. https://doi.org/10.1016/j.neubiorev.2015.08.002
- Cui X, Bray S, Reiss AL. Speeded Near Infrared Spectroscopy (NIRS) Response Detection. PLOS ONE. 2010;5(11):e15474. https://doi.org/10.1371/journal.pone.0015474
- Tong Y, Frederick B deB. Time lag dependent multimodal processing of concurrent fMRI and near-infrared spectroscopy (NIRS) data suggests a global circulatory origin for low-frequency oscillation signals in human brain. NeuroImage. 2010;53(2):553-564. https://doi.org/10.1016/j.neuroimage.2010.06.049
- Vitorio R, Stuart S, Rochester L, Alcock L, Pantall A. fNIRS response during walking – Artefact or cortical activity? A systematic review. Neurosci Biobehav Rev. 2017;83:160-172. https://doi.org/10.1016/j.neubiorev.2017.10.002
- Nicolas-Alonso LF, Gomez-Gil J. Brain Computer Interfaces, a Review. Sensors. 2012;12(2):1211-1279. https://doi.org/10.3390/s120201211
- Herold F, Wiegel P, Scholkmann F, Thiers A, Hamacher D, Schega L. Functional near-infrared spectroscopy in movement science: a systematic review on cortical activity in postural and walking tasks. Neurophotonics. 2017;4(4):041403. https://doi.org/10.1117/1.NPh.4.4.041403
- Suzuki M, Miyai I, Ono T, Kubota K. Activities in the frontal cortex and gait performance are modulated by preparation. An fNIRS study. NeuroImage. 2008;39(2):600-607. https://doi.org/10.1016/j.neuroimage.2007.08.044
- Bishnoi A, Holtzer R, Hernandez ME. Brain Activation Changes While Walking in Adults with and without Neurological Disease: Systematic Review and Meta-Analysis of Functional Near-Infrared Spectroscopy Studies. Brain Sci. 2021;11(3):291. https://doi.org/10.3390/brainsci11030291
- Sui SX, Hendy AM, Teo WP, Moran JT, Nuzum ND, Pasco JA. A Review of the Measurement of the Neurology of Gait in Cognitive Dysfunction or Dementia, Focusing on the Application of fNIRS during Dual-Task Gait Assessment. Brain Sci. 2022;12(8):968. https://doi.org/10.3390/brainsci12080968
- la Fougère C, Zwergal A, Rominger A, et al. Real versus imagined locomotion: A [18F]-FDG PET-fMRI comparison. NeuroImage. 2010;50(4):1589-1598. https://doi.org/10.1016/j.neuroimage.2009.12.060
- FDG PET & Gait. Brain and Movement Newcastle University. Accessed July 12, 2023. https://www.bam-ncl.co.uk/fdg-pet-gait
- Sigurdsson HP, Yarnall AJ, Galna B, et al. Gait‐Related Metabolic Covariance Networks at Rest in Parkinson’s Disease. Mov Disord. 2022;37(6):1222-1234. https://doi.org/10.1002/mds.28977
- Brookes MJ, Leggett J, Rea M, et al. Magnetoencephalography with optically pumped magnetometers (OPM-MEG): the next generation of functional neuroimaging. Trends Neurosci. 2022;45(8):621-634. https://doi.org/10.1016/j.tins.2022.05.008
- Tierney TM, Levy A, Barry DN, et al. Mouth magnetoencephalography: A unique perspective on the human hippocampus. Neuroimage. 2021;225:117443. https://doi.org/10.1016/j.neuroimage.2020.117443
- Lanciego JL, Luquin N, Obeso JA. Functional Neuroanatomy of the Basal Ganglia. Cold Spring Harb Perspect Med. 2012;2(12):a009621. https://doi.org/10.1101/cshperspect.a009621


