
This article is sponsored by Bial and is intended for UK Healthcare Professionals.
Opicapone (ONgentys®) Prescribing Information and Adverse Event reporting can be found at the end of this article.
Adverse events should be reported. Reporting forms and information can be found at: https://yellowcard.mhra.gov.uk/ or in Ireland at:
https://www.hpra.ie. Adverse events should also be reported to BIAL on +44 (0)1628 531171 or bial@pharmalex.com
The catechol-O-methyltransferase (COMT) inhibitor opicapone (ONgentys®) is recommended as an option for use in NHS Wales as adjunctive therapy to preparations of levodopa/DOPA decarboxylase inhibitors (DDCI) in adult patients with Parkinson’s disease (PD) and end-of-dose motor fluctuations who cannot be stabilised on those combinations.1,2 The latest update by the All Wales Medicines Strategy Group (AWMSG) removes the current restriction for opicapone use only after failure of entacapone or in patients who cannot tolerate entacapone, which is similar to the positioning by the Scottish Medicines Consortium and Northern Ireland (NI) formulary recommendations alongside some of the integrated care systems (ICS) in England.1,3–8
Ensuring wider access to treatments across the United Kingdom
Uptake of innovative medicines remains variable across the UK, which can be attributed to delayed access to and participation in clinical trials, varied uptake of treatments approved by NICE and its devolved nation equivalents by local NHS systems, and inequitable patient access to treatment.9 Addressing the challenges at a local and national level could improve patient outcomes and ensure equitable access to treatment.9 NHS England has published guidance for integrated care boards and regional leadership teams on the roles, responsibilities and accountabilities for delivering medicines optimisation in England.10 The latest update by the AWMSG means there is now equitable access to opicapone in Wales, Scotland, NI and some ICS in England within its licensed indication,1–8 providing clinicians with an alternative treatment option to the current available armamentarium to support patients experiencing motor fluctuations due to levodopa wearing-off as early as possible.
Opicapone: evidence from the exploratory ADOPTION study for early add-on therapy11
The eArly L-Dopa with Opicapone in Parkinson’s paTients wIth motOr fluctuatioNs (ADOPTION) study was a randomised, parallel-group, open-label, exploratory Phase 4 study conducted in Korea.11 A total of 169 participants (aged ≥30 years) with idiopathic PD treated with 3–4 daily oral levodopa doses up to
600 mg for ≥4 weeks prior to study entry and demonstrating signs of wearing-off (average of total daily OFF‑time ≥1 hour for ≥4 weeks but <2 years) were randomised (1:1) to opicapone 50 mg once daily or 100 mg levodopa/DDCI (added to the current levodopa/DDCI therapy) for 4 weeks.11 The main efficacy endpoint was change from baseline to end of study in absolute OFF‑time.11 Endpoints also included changes in ON‑time.11
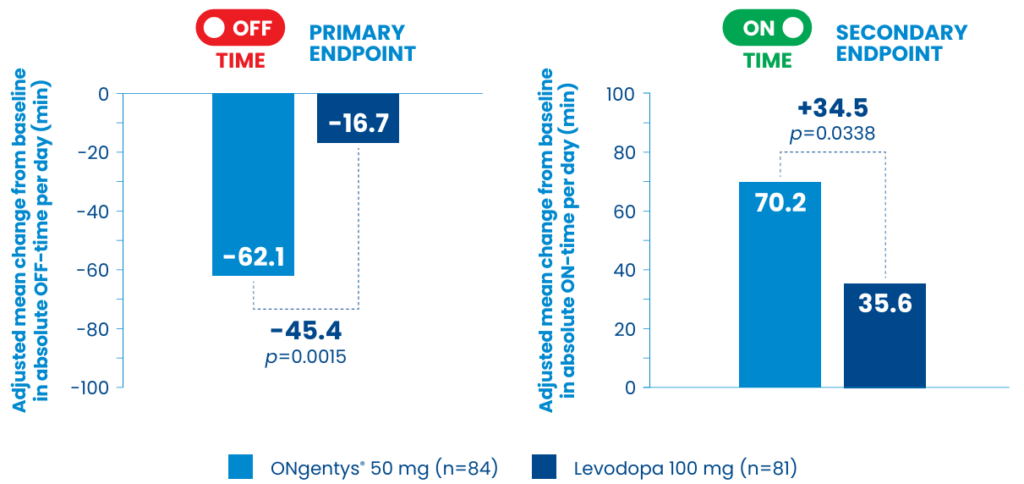
Efficacy: Once-daily opicapone was significantly more effective in improving OFF- and ON-time than an additional 100 mg of levodopa in patients who experienced early wearing-off11 meaning more time for them to be active in the day.
Safety profile: Opicapone was a well-tolerated strategy to treat early wearing-off.11The percentage of participants who reported adverse events (AEs) from the exploratory ADOPTION study was higher for opicapone vs the levodopa group (37.9% vs 18.5%; n/N=33/87 and n/N=15/81, respectively), with dizziness (9.2% vs 3.7%; n/N=8/87 and n/N=3/81, respectively) and dyskinesia (8.1% vs 1.2%; n/N=7/87 and n/N=1/81, respectively) being the most commonly reported AEs.11 The incidence of AEs leading to discontinuation was low (<5%) and similar between groups (3.5% with opicapone vs 2.5% with levodopa; n/N=3/87 and n/N=2/81, respectively).11 The safety profile of opicapone in the ADOPTION study was consistent with its Summary of Product Characteristics, with no new undesirable effects reported, although dyskinesia appeared to be less common in the ADOPTION trial (8.1% incidence) than in placebo-controlled Phase 3 studies (17.7% incidence).2,11
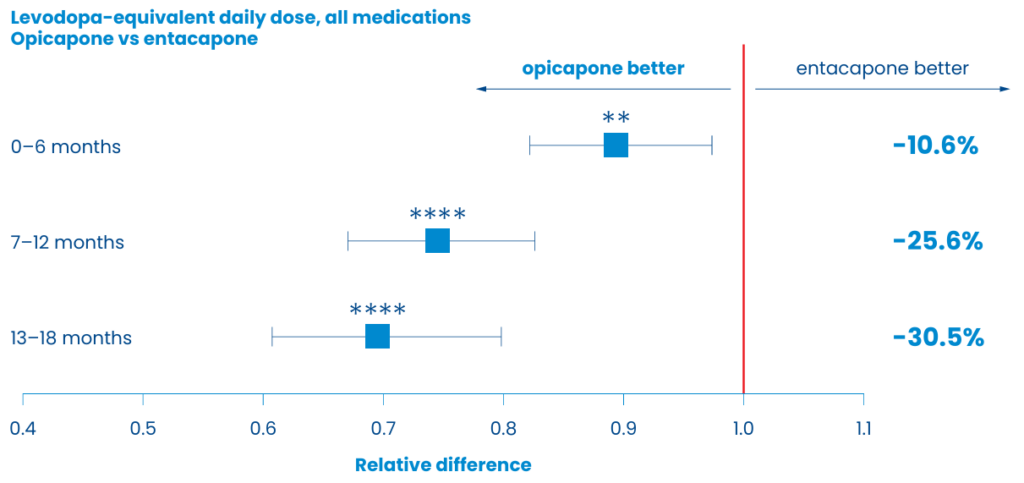
A retrospective cohort study of real-world UK data using electronic health records compared the effect of first-line opicapone (n=173) with entacapone (n=433) on healthcare resource utilisation (HCRU).12 This retrospective study demonstrated that opicapone is likely to decrease the need for post-treatment HCRU (such as reduced hospital touch points, ie, fewer neurology and general outpatient visits at 6 months maintained for up to 18 months of treatment, established using Hospital Episode Statistics data for England) vs entacapone.12
Furthermore, opicapone was associated with a progressive reduction of levodopa-equivalent daily dose (LEDD) vs initiation of entacapone. Regression analyses showed that opicapone had a 30.5% lower LEDD at 18 months vs entacapone (p=0.0001).12
In summary
- There is now equitable access to opicapone in Wales, Scotland, NI and some ICS in England within its licensed indication, providing clinicians with a greater choice of treatment options to optimise the management of people with PD experiencing end-of-dose motor fluctuations with levodopa and DDCI1–8,13
- The ADOPTION study explored the clinically relevant question of which approach is more efficacious in improving early wearing-off symptoms, and supports opicapone as an effective first-line alternative to levodopa adjustment-only strategies11
- A retrospective data-based comparison of electronic health records in the UK demonstrated that opicapone is likely to decrease the need for post-treatment HCRU via fewer neurology and general outpatient visits in England as well as lowering the LEDD compared with entacapone12
References
- All Wales Therapeutics and Toxicology Centre. AWMSG Secretariat assessment report. Opicapone (Ongentys®) 25 mg and 50 mg hard capsules. Reference number: 5285. April 2024. Available at: https://awttc.nhs.wales/files/appraisals-asar-far/appraisal-report-opicapone-ongentys-5285/. Accessed: October 2024.
- Ongentys 50 mg hard capsules. Summary of Product Characteristics. Bial Pharma UK Ltd. Updated 24-Nov-2022.
- Scottish Medicines Consortium. Opicapone (Ongentys®). Available at: https://tinyurl.com/fchsp9ap. Accessed: October 2024.
- Northern Ireland Formulary. Managed entry decisions. Opicapone (Ongentys®). Available at: https://niformulary.hscni.net/managed-entry/managed-entry-decisions/. Accessed: October 2024.
- NHS Nottinghamshire Area Prescribing Committee Formulary. Available at: Nottinghamshire Area Prescribing Committee Formulary (nottinghamshireformulary.nhs.uk). Accessed: October 2024.
- NHS North East and North Cumbria ICS Formulary. Available at: North East and North Cumbria Formulary (northeastnorthcumbriaformulary.nhs.uk). Accessed: October 2024.
- NHS Hampshire and Isle of Wight Formulary. Available at: NHS Hampshire and Isle of Wight Formulary (hiowformulary.nhs.uk). Accessed: October 2024.
- NHS Cornwall and Isles of Scilly Formulary. Available at: eclipsesolutions.org/cornwall/info.aspx?paraid=96. Accessed: October 2024.
- A report from the Patient Advisory Council and the ABPI. How to make sure patients get faster, more equitable access to innovative treatments. February 2024. MA-0167-0124. Available at: abpi_patient_advisory_council_report_feb-2024.pdf. Accessed: October 2024.
- NHS England. Regional arrangements for medicines optimisation in the NHS in England. Guidance for integrated care boards and NHS England regional leadership teams. Published: July 2023. Available at: https://tinyurl.com/4a8sfndw. Accessed: October 2024.
- Lee JY et al. Opicapone to treat early wearing-off in Parkinson’s disease patients: the Korean ADOPTION trial. Mov Disord Clin Pract 2024;11(6):655–65.
- Harrison-Jones G et al. Opicapone versus entacapone: head-to-head retrospective data-based comparison of healthcare resource utilization in people with Parkinson’s disease new to catechol-O-methyltransferase (COMT) inhibitor treatment. Eur J Neurol 2023;30(10):3132–41.
- Ferreira JJ et al. Effect of opicapone on levodopa pharmacokinetics in patients with fluctuating Parkinson’s disease. Mov Disord 2022;37(11):2272–83.
Adverse events should be reported. Reporting forms and information can be found at: https://yellowcard.mhra.gov.uk/ or in Ireland at:
https://www.hpra.ie. Adverse events should also be reported to BIAL on +44 (0)1628 531171 or bial@pharmalex.com
UK/ON/2024/073
Date of preparation: October 2024
Click here for Prescribing Information
Bial Pharma UK Ltd, Admiral House, St Leonards Road, Windsor, Berkshire SL4 3BL