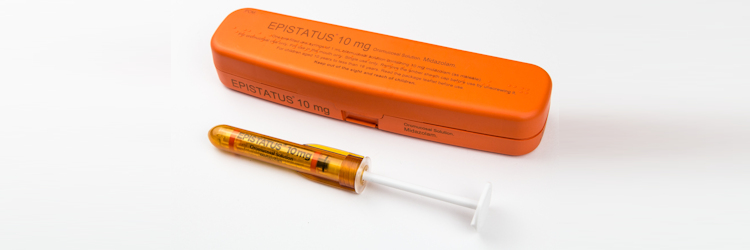
Epistatus is licensed for use in the treatment of prolonged, acute convulsive seizures in children and adolescents aged 10 to less than 18 years, who have been diagnosed with epilepsy1. Epistatus is presented “ready-to-use” in a novel, pre-filled, single-dose syringe, in a volume of 1 mL, to provide carers with the confidence that they are administering the correct dose1,2.
Epistatus has been developed specifically for buccal administration. The pre-filled syringe comes in a robust, tamper-resistant pack, which is UV-resistant to maximise shelf life1. It is portable and is carried by your patients at all times2.
The single-dose pack meets the principles of the Medicines Optimisation and NHS RightCare approach to help reduce wastage3and is compliant with the Falsified Medicines Directive that will come into force in February 20194.The NHS price for a single 10mg in 1mL pre-filled syringe is £45.76.
Please visit www.epistatus.co.uk for further information about Epistatus 10mg oromucosal midazolam pre-filled syringe, or to find out more about a budget model to calculate the financial impact of prescribing multipack buccal midazolam versus single-pack Epistatus.
Prescribing information can be found at https://www.epistatus.co.uk/downloads/epistatus_summary_of_product_characteristics.pdf
References:
1) Epistatus 10mg oromucosal solution. Summary of Product Characteristics.
2) Data on file – Excerpts from Epistatus Patent Application.
3) Medicines Optimisation: Helping patients to make the most of medicines. Royal Pharmaceutical Society May 2013.
4) Pharmaceutical waste reduction in the NHS. NHS England, June 2015.
