
This article was submitted by Otto Bock Healthcare and they have sponsored its publication in ACNR. Dr Michael Jauch is clinical partner to Otto Bock Healthcare UK for Functional Electrostimulation. Dr Jauch graduated in Germany. He undertook postgraduate training in orthopaedics in Germany and the UK as well as training in orthopaedic and neurological rehabilitation in Germany. His Postgraduate work in experimental orthopaedics was undertaken at Imperial College, London. Since 2011 he has operated an FES clinic at BMI Blackheath Hospital, London.
The ability to negotiate the environment independently is fundamental to all aspects of daily life and almost all aspects of social participation are dependent upon adequate mobility. The insufficiency in dorsiflexion during gait results in difficulties in walking, such as slowness, tripping and tiredness1-3, leading to a reduction in mobility and independence as well as increased risk of falls.” NICE 4.
For many patients who suffer from central or upper motor neuron lesions, e.g. stroke, multiple scleroris or head injury, walking becomes a challenging task. In many cases, the damage to the central nervous system results in paralysis and a drop foot.
This article concerns CNS lesions. Lesions to peripheral nerves are an exclusion criteria for the application of Functional Electrical Stimulation (FES).
Walking speed has been shown to be a clinically relevant outcome. Some researchers even considered it to be the ‘almost perfect’ measure of community ambulation5. Reduced gait speed was shown to be related to the increased risk of future hospitalisation, future lower extremity limitation and even mortality.
 National Guidance
National Guidance
Since the introduction of FES as a drop foot treatment, a number of studies have demonstrated that FES significantly improved walking speed and patient’s quality of life6-8. These health and quality of life benefits, particularly improved independence, are in line with the goals of the Department of Health Reablement initiatives.
NICE published interventional procedural guidance on FES in 20093 stating that the current evidence on the safety and efficacy (in terms of improving gait) of FES for drop foot of central neurological origin appears adequate to support the use of this procedure provided that normal arrangements are in place for clinical governance, consent and audit. For implantable devices, an interdisciplinary healthcare team should be involved in deciding which patients should have the procedure3.
Other guidance includes the government’s National Stroke Strategy. It acknowledges FES as a new technology with which service providers need to keep pace9.
The National Service Framework for long term conditions includes a quality requirement (QR7) advocating appropriate assistive technology/equipment10.
In a 2010 report, the effectiveness and cost-effectiveness of surface FES as treatment for drop foot was examined. The conservative model on the use of surface FES to treat drop foot after stroke shows that it is likely to be cost effective compared to no treatment. This report suggests that it is reasonable to assume that the QALY gain may be higher for implantable systems11.
The National clinical guideline for stroke (ICSWP) 2012 of the Royal College of Physicians12 differentiates between Therapeutic Electrical Stimulation (TES) which long-term use aims to improve recovery of function vs Functional Electrical Stimulation for immediate functional improvement. It concludes that so far the findings of RCTs and papers about therapeutic electrical stimulation are contradictory regarding impairment and activity and that there are so far no cost-effectiveness studies in this area. It therefore recommends to use TES only in the context of clinical trials. However, FES can be used where arrangements for clinical governance, audit and consent are in place.
Treatment options
Ankle-Foot Orthosis (AFO)
Conventional treatment options for drop foot are primarily physiotherapy and the use of an AFO. AFOs aim to support the foot and ankle, but as it is a passive device, it will not activate the users’ own muscles to enhance walking. Additionally, medical therapy (such as baclofen and botulinum toxin) or surgery for refractory cases (tendon transfer, arthrodesis) may sometimes be used3,13.
Surface FES
Surface electrodes are applied over the common peroneal nerve in the area of the head of the fibula and a battery-powered stimulator which is controlled by a foot switch or sensor provides timed stimulation of nerve/muscle from heel lift to heel strike, providing the necessary foot lift during the swing phase of the gait cycle.
Clinical studies evaluating the effectiveness of drop foot stimulation suggest that it provides many benefits to patients, such as an improved confidence in walking, increased walking speed and endurance, less effort during walking and reduced spasticity. Additional benefits are related to a potential reduction in the risk of falling 14-18.
The FES systems available nowadays have developed considerably since their introduction in 1961, but still some technical side effects are observed, such as the lack of selectivity of muscle recruitment to electrode placement, as well as pain, tissue irritation and possible skin damage associated with the passage of current through the skin17. Taylor et al. identified problems with locating the electrodes for effective stimulation as the most common non-physiological reason for discontinuing the use of the surface stimulator18. Electrode positioning becomes even more of an issue for patients with upper limb impairment.
Surface stimulators currently available are:
Pace by Odstock: A wired system containing a pocket sized control unit, self adhesive skin electrodes and a wired foot switch.
WalkAide by Trulife: A self-contained system which contains surface electrodes, control unit and an inertial gait sensor within one cuff to be worn immediately below the knee.
L300 by Bioness: A cuff based system worn below the knee. A control unit and foot switch are worn separately from the cuff and communicate wirelessly.
MyGait by Otto Bock Healthcare: the newest surface stimulator launched in 2013. A cuff-based unit with the stimulator worn in the cuff linked wirelessly to a foot switch and a patient remote control. The novelty of this stimulator is that it provides two channels of stimulation to combine two different muscle groups where indicated.
Implantable FES
A newer alternative to surface stimulators are implantable devices (the StimuStep from Finetech Medical and the ActiGait from Otto Bock), which overcome most of the problems encountered with surface stimulators and thus are more desirable for the following reasons:
- Electrodes are surgically implanted, and hence no surface electrodes required
- Optimal electrode position achieved and controlled during surgical implantation
- No need for technically challenging electrode placement by patient lengthening daily set-up [13]
- No soft tissue or skin reactions
- No discomfort / pain due to constant electrical sensation through the skin. Improved ease of use and cosmetic appearance.
New Developments
The main new development for surface stimulation is the launch of the MyGait, the first two channel wireless surface stimulator, in early 2013. Clinical follow-up studies will be carried out in time. First results from pre-launch field studies (based on 17 patients) showed that 12 out of 17 patients preferred MyGait over their previous or other fitting, 57% of patients felt MyGait to be an improvement over their previous system.19
Indications for MyGait are stroke, cranio- cerebral injury, multiple sclerosis, Incomplete spinal cord injury and infantile cerebral palsy.
Implanted stimulators are still a new development in themselves. Both StimuStep and ActiGait have been implanted in a number of European countries in recent years. ActiGait was launched in the UK in late 2011 in our clinic. Main indication for implanted devices is drop foot secondary to stroke. There are suggestions of benefit for other upper motor neuron conditions, but still with lack of scientific support and regulatory issues.
StimuStep
An implanted system with electrodes (2 channels) imbedded into the epineurium of the common peroneal nerve’s deep and superficial branches. The implant receiver under the skin receives power and control signals from the control unit, which is triggered by a footswitch. The control unit is worn on a belt below the knee and needs to be positioned on top of the below skin receiver unit . Communication between the external control unit and the footswitch is wired.
ActiGait
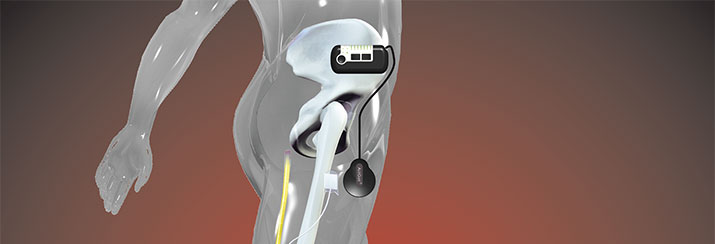
The system consists of a heel switch (3 in diagram above) which communicates wirelessly with a control unit (1), which is worn on a magnetic clip anywhere discreet as chosen by the patient. This unit allows the patient to adjust the intensity of the stimulation. An electromagnetic signal is painlessly sent through the skin at the upper thigh via a lightweight antenna (2) to the implant (4), which converts that signal into electric current for the 4 channel electrode cuff positioned around the Common Peroneal Nerve. The four channels can be programmed to allow for selective nerve bundle stimulation and balanced dorsiflexion / eversion.
Review ActiGait vs StimuStep
A clinical follow-up (a mixed population of 46 cases since 2006 of which 42 were reviewed for the study) of StimuStep was presented by Taylor20,21. The StimuStep users were selected from existing surface FES users. Reasons for selection of the implant were skin irritation, patients’ difficulties with electrode placement or anticipated long term use. Indications were stroke (18 cases), MS (17 cases, 1 bilateral), traumatic head injury (3), incomplete spinal cord injury (2), brain tumour (1), Parkinson’s (1), transverse myelitis (2) and cerebral palsy (1). 4 patients were not followed up due to : 2 non-functioning implants, 1 explantation because of infection and 1 for poor response because of abnormal nerve anatomy. The main benefits to patients reported were improvements in walking speed (18%) and a three-minute walking distance (23%).
Complications were reported as 6 electrode failures, 9 cases of nerve dysfunction (likely due to epineural electrode positioning and direct pressure on the receiver). Electrical sensation only improved 1 point out of 10 in comparison to surface stimulation with two cases even more uncomfortable level of sensation than surface stimulation. Five cases of skin reaction were reported. The patient still needs to wear a cuff directly on the skin which has a large contact area and some contact pressure. Despite implanted device patients still experienced issues with electrode and control box position. 11 of the first 16 cases also had reliability problems with the stimulation channel to the superficial branch of the nerve.
ActiGait was the subject of a safety and performance study conducted in three centres in Denmark1, which established safety using nerve conduction velocity and performance improvements in walking speed (20%) and distance walked in four minutes (14%). Long-term improvements were detected in walking speed and distance when stimulated, and the orthotic effect of stimulation showed statistically significant improvement. Furthermore, qualitative responses highlighted improvement in confidence with less fear of falling, promoting the long-term potential to provide a positive effect on personal well-being, safety and performance1,8. Similar patient benefits were reported in a more recent study22 showing a 24.5% increase in walking speed, and 17% increase in walking distance in the six-minute walking test. In addition to walking speed and endurance, the kinematic and biomechanical changes were investigated in five subjects by Ernst et al23. The study demonstrated a restored ankle joint movement towards a more physiological pattern as seen in normal gait.
The ActiGait implant complication rate was followed up by the manufacturer’s internal quality control24. Since the introduction of the newest revision of the device in February 2011, 115 implantations were reviewed (mixed population, indication stroke). All reported complications had been operator-caused (surgical procedure / general surgical risk), none have been caused by the implant. The complications reported were 4 cases of infection and 4 cases of temporary nerve damage (3 of which are functional with ActiGait in situ at the time of writing). Surgical reasons were established as causes for those nerve damages. Since the last revision of the surgical procedure 60 implantations have been carried out with no nerve damages, no implant failures nor incidents with external components. For licensing reasons the main indication for ActiGait is stroke. However, first implantations have been carried out for alternative indications in our practice, such as head injury and multiple scleroris with very good individual outcomes in the improvement of quality of life. Further work will be undertaken in implantations for other indications.
It appears that by direct comparison the benefits of both implants are compatible. However, comparing the reported complications, ActiGait has despite higher case numbers a lower complications rate, none of which are caused by the device itself.
References
1.Burridge JH, Haugland M, Larsen B, Pickering RM, Svaneborg N, Iversen HK, et al. Phase II trial to evaluate the ActiGait implanted drop-foot stimulator in established hemiplegia. Journal of Rehabilitation Medicine, 2007;39(3):212-8.
2.Burridge J, Taylor P, Hagan S, Wood D, Swain I. The effect of common peroneal nerve stimulation on quadriceps spasticity in hemiplegia. Physiotherapy, 1997;83(2):82-9.
3.National Institute for Health and Clinical Excellence. Interventional procedures overview 278: Functional electrical stimulation for drop foot of central neurological origin. 2009.
4.National Institute for Health and Clinical Excellence. Clinical guideline 8: Multiple Sclerosis; National clinical guideline for diagnosis and management in primary and secondary care. 2004.
5.Wade D. Measurement in Neurological Rehabilitation. Oxford, UK: Oxford University Press; 1992.
6.Kottink AI, IJzerman MJ, Groothuis-Oudshoorn CG, Hermens HJ. Measuring Quality of Life in Stroke Subjects Receiving an Implanted Neural Prosthesis for Drop Foot. Artificial Organs, 2010;34(5):366-76.
7.Barrett C, Taylor P. The Effects of the Odstock Drop Foot Stimulator on Perceived Quality of Life for People With Stroke and Multiple Sclerosis. Neuromodulation: Technology At The Neural Interface. 2010;13(1):58-64.
8.Burridge JH, Haugland M, Larsen B, Pickering RM, Svaneborg N, Iversen HK, et al. Patients’ perceptions of the benefits and problems of using the ActiGait implanted drop foot stimulator. Journal of Rehabilitation Medicine. 2008;40:873-5.
9.Department of Health. National Stroke Strategy. 2007.
10.Department of Health. National Service Framework for long term conditions. 2005.
11.CEP10012: Centre for evidence-based Purchasing. Economic report: functional electrical stimulation for drop foot of central neurological origin. NHS Purchasing and Supply Agency. 2010. London.
12.Intercollegiate Stroke Working Party. National Clinical Guideline for Stroke, 4th edition London: Royal College of Physicians 2012. ISBN 9781860164927
13.CEP10010: Centre for evidence-based Purchasing. Buyer’s guide: functional electrical stimulation for drop foot of central neurological origin. NHS Purchasing and Supply Agency. 2010. London.
14.Mann GE, Jolley CL, Taylor PN. An Investigation into the effect of functional electrical stimulation on mobility and quality of life in patients with multiple sclerosis. Proceedings of the 10th Annual Conference of the International FES Society. July 2005, Canada.
15.Daly J, Roenigk K, Holcomb J, Rogers JM, Butler K, Gansen J, McCabe J, Fredrickson E, Marsolais E, Ruff R. A randomized controlled trial of functional neuromuscular stimulation in chronic stroke subjects. Stroke, 2006, 37(1):172-8.
16.van Swigchem R, Weerdesteyn V, van Duijnhoven HJ, den Boer J, Beems T, Geurts AC. Near-Normal Gait Pattern With Peroneal Electrical Stimulation as a Neuroprosthesis in the Chronic Phase of Stroke: A Case Report. Arch Phys Med Rehabil, 2011, 92:320-4.
17.Waters RL, McNeal D, Perry J. Experimental correction of foot drop by electrical stimulation of the peroneal nerve. Journal of Bone Joint Surgery Am, 1975, 57:1047-1054.
18.Taylor PN, Burridge JH, Dunkerley AL, Lamb A, Wood DE, Norton JA, Swain ID. Patients’ perceptions of the Odstock Dropped Foot Stimulator (ODFS). Clinical Rehabilitation, 1999, 13:439-446.
19.Otto Bock Healthcare, Internal Report: MyGait Field Test Results, April 2013.
20. Taylor P, Wilkinson IH, Humphreys L, Kwan Y, Slade-Sharman D, Khan M, Hobby J. Clinical Experience of the STIMuSTEP Implated Dropped Foot Stimulator. International IFESS Conference 2012, Banff, Alberta, Canada.
21.Taylor P, Wilkinson I, Samuel V et al. A comparison of external and implanted EFS for correction of dropped foot. An audit of the STIMuSTEP service in Salisbury. 4th Annual UKRI IFESS Conference 2013.
22.Rohde V, Wachter D, Ernst J, Liebetanz D. Perneal stimulation for foot drop management after chronic stroke: Experience in 25 Patients, 63. Jahrestagung der Deutschen Gesellschaft fuer Neurochirurgie, 2012, Leipzig.
23.Ernst J, Grundeya J, Hewitta M, von Lewinskia F, Kaus J, Schmalz T, Rohde V, Liebetanz D. Towards physiological ankle movements with the ActiGait implantable drop foot stimulator in chronic stroke. Restorative Neurology and Neuroscience. In Press.
24.Statement about ActiGait Complication Rates by Dr. Andreas Hahn (Managing Director, nStim Services) 16 April 2013.
ACNR 2013;13:5:26-28

